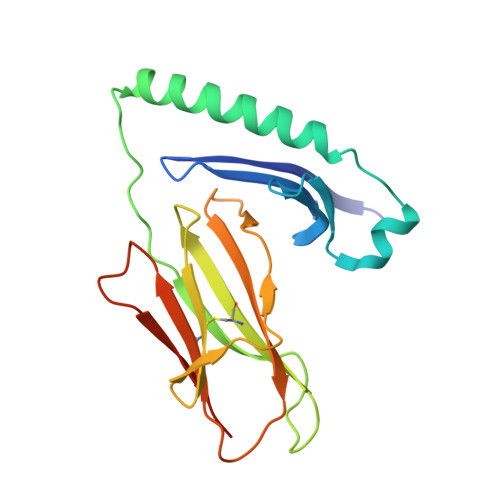
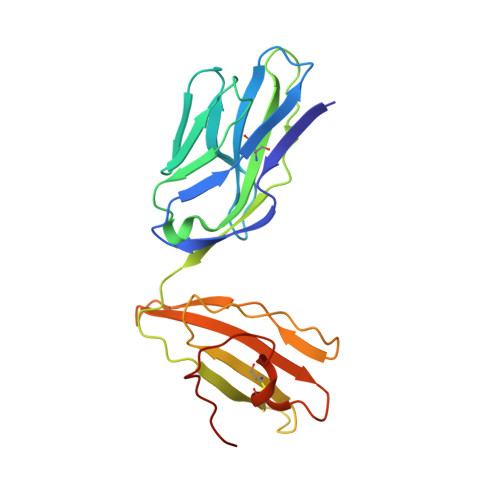
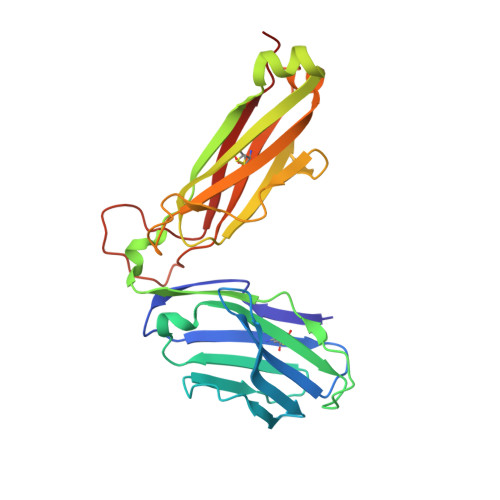
The shared susceptibility epitope of HLA-DR4 binds citrullinated self-antigens and the TCR.
Lim, J.J., Jones, C.M., Loh, T.J., Ting, Y.T., Zareie, P., Loh, K.L., Felix, N.J., Suri, A., McKinnon, M., Stevenaert, F., Sharma, R.K., Klareskog, L., Malmstrom, V., Baker, D.G., Purcell, A.W., Reid, H.H., La Gruta, N.L., Rossjohn, J.(2021) Sci Immunol 6
- PubMed: 33863750
- DOI: https://doi.org/10.1126/sciimmunol.abe0896
- Primary Citation of Related Structures:
6V0Y, 6V13, 6V15, 6V18, 6V19, 6V1A - PubMed Abstract:
Individuals expressing HLA-DR4 bearing the shared susceptibility epitope (SE) have an increased risk of developing rheumatoid arthritis (RA). Posttranslational modification of self-proteins via citrullination leads to the formation of neoantigens that can be presented by HLA-DR4 SE allomorphs. However, in T cell-mediated autoimmunity, the interplay between the HLA molecule, posttranslationally modified epitope(s), and the responding T cell repertoire remains unclear. In HLA-DR4 transgenic mice, we show that immunization with a Fibβ-74cit 69-81 peptide led to a population of HLA-DR4 Fibβ-74cit69-81 tetramer + T cells that exhibited biased T cell receptor (TCR) β chain usage, which was attributable to selective clonal expansion from the preimmune repertoire. Crystal structures of pre- and postimmune TCRs showed that the SE of HLA-DR4 represented a main TCR contact zone. Immunization with a double citrullinated epitope (Fibβ-72,74cit 69-81 ) altered the responding HLA-DR4 tetramer + T cell repertoire, which was due to the P2-citrulline residue interacting with the TCR itself. We show that the SE of HLA-DR4 has dual functionality, namely, presentation and a direct TCR recognition determinant. Analogous biased TCR β chain usage toward the Fibβ-74cit 69-81 peptide was observed in healthy HLA-DR4 + individuals and patients with HLA-DR4 + RA, thereby suggesting a link to human RA.
- Infection and Immunity Program and Department of Biochemistry and Molecular Biology, Biomedicine Discovery Institute, Monash University, Clayton, Victoria 3800, Australia.
Organizational Affiliation: