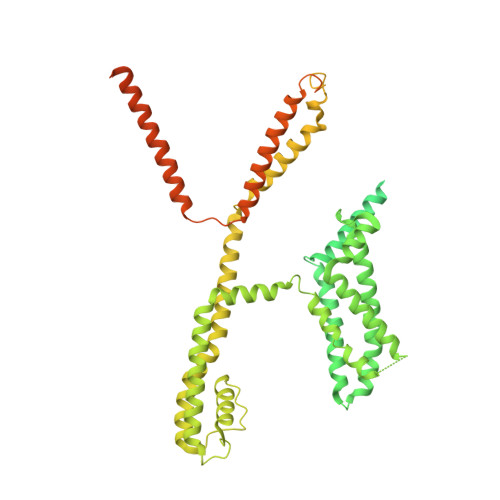
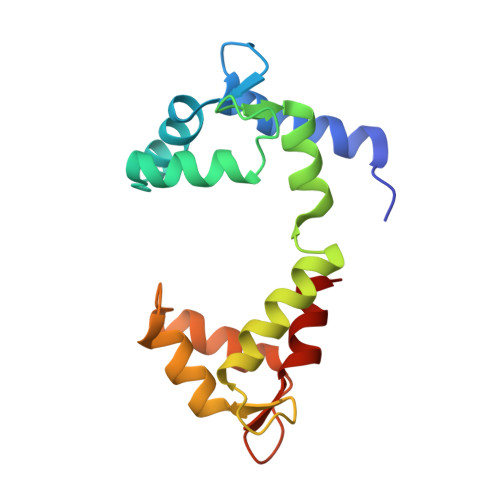
Structural Basis for the Modulation of Human KCNQ4 by Small-Molecule Drugs.
Li, T., Wu, K., Yue, Z., Wang, Y., Zhang, F., Shen, H.(2021) Mol Cell 81: 25
- PubMed: 33238160
- DOI: https://doi.org/10.1016/j.molcel.2020.10.037
- Primary Citation of Related Structures:
7BYL, 7BYM, 7BYN - PubMed Abstract:
Among the five KCNQ channels, also known as the K v 7 voltage-gated potassium (K v ) channels, KCNQ2-KCNQ5 control neuronal excitability. Dysfunctions of KCNQ2-KCNQ5 are associated with neurological disorders such as epilepsy, deafness, and neuropathic pain. Here, we report the cryoelectron microscopy (cryo-EM) structures of human KCNQ4 and its complexes with the opener retigabine or the blocker linopirdine at overall resolutions of 2.5, 3.1, and 3.3 Å, respectively. In all structures, a phosphatidylinositol 4,5-bisphosphate (PIP 2 ) molecule inserts its head group into a cavity within each voltage-sensing domain (VSD), revealing an unobserved binding mode for PIP 2 . Retigabine nestles in each fenestration, inducing local shifts. Instead of staying within the central pore, linopirdine resides in a cytosolic cavity underneath the inner gate. Electrophysiological analyses of various mutants corroborated the structural observations. Our studies reveal the molecular basis for the modulatory mechanism of neuronal KCNQ channels and provide a framework for structure-facilitated drug discovery targeting these important channels.
- Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, Hangzhou, Zhejiang 310024, China; Westlake Laboratory of Life Sciences and Biomedicine, Hangzhou, Zhejiang 310024, China; Institute of Biology, Westlake Institute for Advanced Study, Hangzhou, Zhejiang 310024, China.
Organizational Affiliation: