ARF GTPases activate Salmonella effector SopF to ADP-ribosylate host V-ATPase and inhibit endomembrane damage-induced autophagy.
Xu, Y., Cheng, S., Zeng, H., Zhou, P., Ma, Y., Li, L., Liu, X., Shao, F., Ding, J.(2022) Nat Struct Mol Biol 29: 67-77
- PubMed: 35046574
- DOI: https://doi.org/10.1038/s41594-021-00710-6
- Primary Citation of Related Structures:
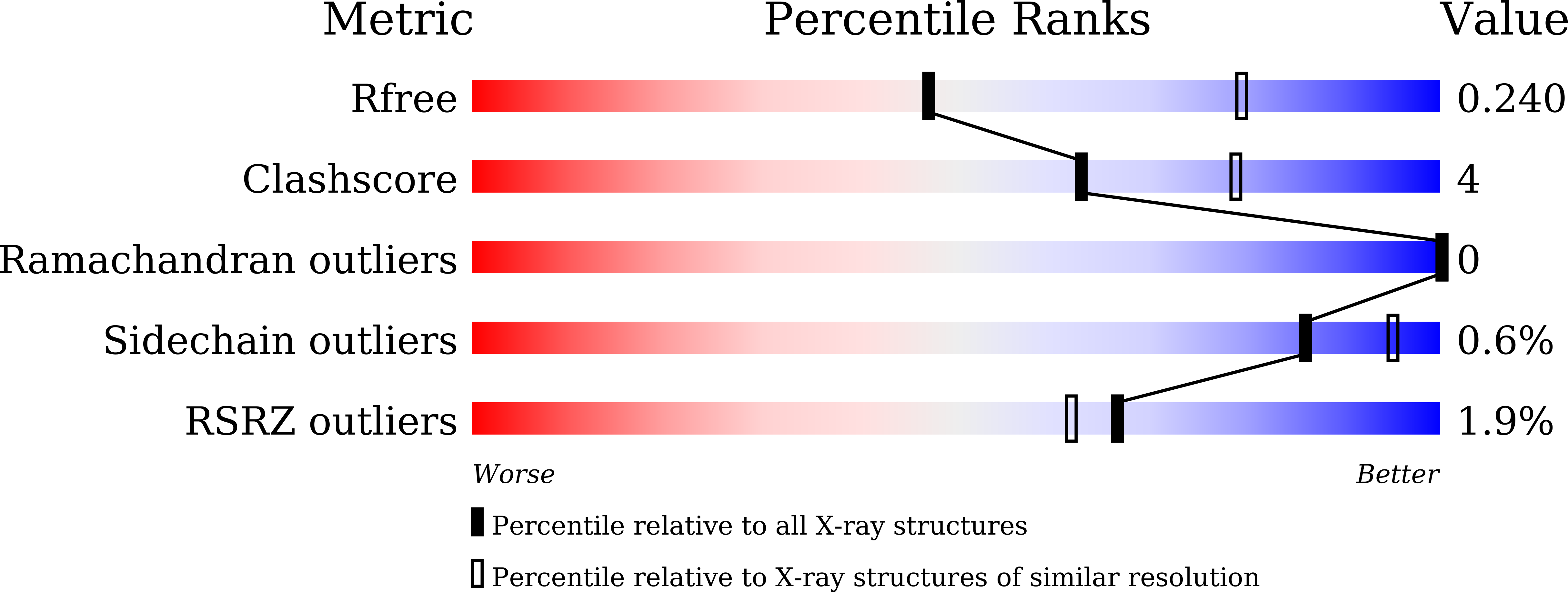
7DN8, 7DN9 - PubMed Abstract:
Selective autophagy helps eukaryotes to cope with endogenous dangers or foreign invaders; its initiation often involves membrane damage. By studying a Salmonella effector SopF, we recently identified the vacuolar ATPase (V-ATPase)-ATG16L1 axis that initiates bacteria-induced autophagy. Here we show that SopF is an ADP-ribosyltransferase specifically modifying Gln124 of ATP6V0C in V-ATPase. We identify GTP-bound ADP-ribosylation factor (ARF) GTPases as a cofactor required for SopF functioning. Crystal structures of SopF-ARF1 complexes not only reveal structural basis of SopF ADP-ribosyltransferase activity but also a unique effector-binding mode adopted by ARF GTPases. Further, the N terminus of ARF1, although dispensable for high-affinity binding to SopF, is critical for activating SopF to modify ATP6V0C. Moreover, lysosome or Golgi damage-induced autophagic LC3 activation is inhibited by SopF or Q124A mutation of ATP6V0C, thus also mediated by the V-ATPase-ATG16L1 axis. In this process, the V-ATPase functions to sense membrane damages, which can be uncoupled from its proton-pumping activity.
Organizational Affiliation:
National Institute of Biological Sciences, Beijing, China.