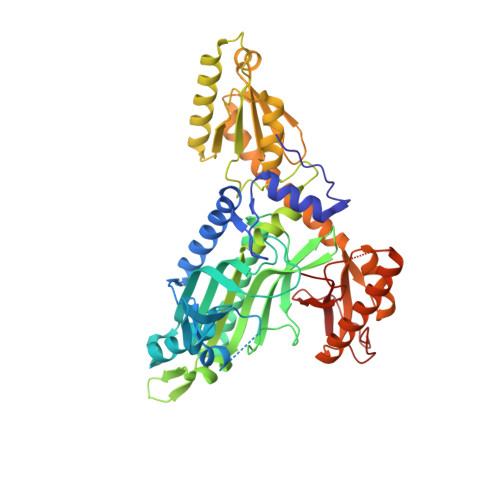
Double drugging of prolyl-tRNA synthetase provides a new paradigm for anti-infective drug development.
Manickam, Y., Malhotra, N., Mishra, S., Babbar, P., Dusane, A., Laleu, B., Bellini, V., Hakimi, M.A., Bougdour, A., Sharma, A.(2022) PLoS Pathog 18: e1010363-e1010363
- PubMed: 35333915
- DOI: https://doi.org/10.1371/journal.ppat.1010363
- Primary Citation of Related Structures:
7EVU, 7EVV - PubMed Abstract:
Toxoplasmosis is caused by Toxoplasma gondii and in immunocompromised patients it may lead to seizures, encephalitis or death. The conserved enzyme prolyl-tRNA synthetase (PRS) is a validated druggable target in Toxoplasma gondii but the traditional 'single target-single drug' approach has its caveats. Here, we describe two potent inhibitors namely halofuginone (HFG) and a novel ATP mimetic (L95) that bind to Toxoplasma gondii PRS simultaneously at different neighbouring sites to cover all three of the enzyme substrate subsites. HFG and L95 act as one triple-site inhibitor in tandem and form an unusual ternary complex wherein HFG occupies the 3'-end of tRNA and the L-proline (L-pro) binding sites while L95 occupies the ATP pocket. These inhibitors exhibit nanomolar IC50 and EC50 values independently, and when given together reveal an additive mode of action in parasite inhibition assays. This work validates a novel approach and lays a structural framework for further drug development based on simultaneous targeting of multiple pockets to inhibit druggable proteins.
Organizational Affiliation:
Molecular Medicine-Structural Parasitology Group, International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India.