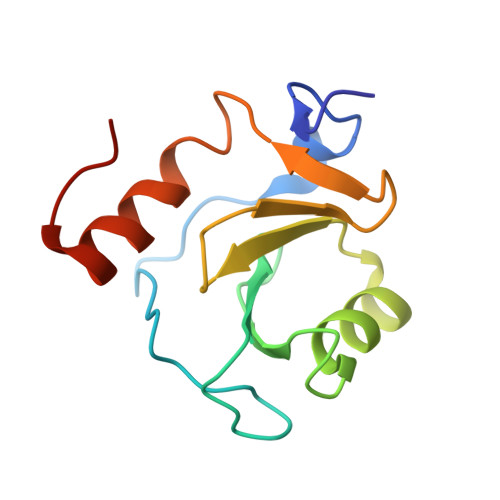
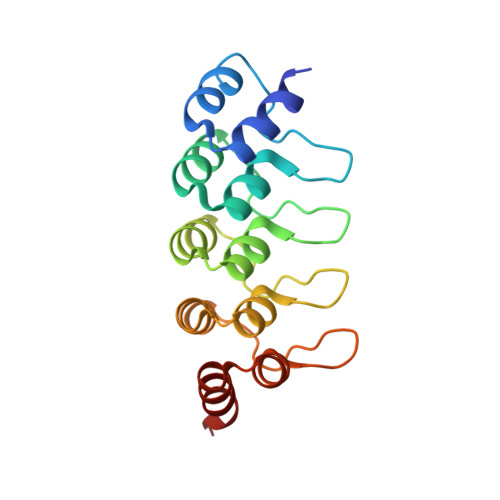
Disrupting the HDAC6-ubiquitin interaction impairs infection by influenza and Zika virus and cellular stress pathways.
Wang, L., Moreira, E.A., Kempf, G., Miyake, Y., Oliveira Esteves, B.I., Fahmi, A., Schaefer, J.V., Dreier, B., Yamauchi, Y., Alves, M.P., Pluckthun, A., Matthias, P.(2022) Cell Rep 39: 110736-110736
- PubMed: 35476995
- DOI: https://doi.org/10.1016/j.celrep.2022.110736
- Primary Citation of Related Structures:
7ZYU - PubMed Abstract:
The deacetylase HDAC6 has tandem catalytic domains and a zinc finger domain (ZnF) binding ubiquitin (Ub). While the catalytic domain has an antiviral effect, the ZnF facilitates influenza A virus (IAV) infection and cellular stress responses. By recruiting Ub via the ZnF, HDAC6 promotes the formation of aggresomes and stress granules (SGs), dynamic structures associated with pathologies such as neurodegeneration. IAV subverts the aggresome/HDAC6 pathway to facilitate capsid uncoating during early infection. To target this pathway, we generate designed ankyrin repeat proteins (DARPins) binding the ZnF; one of these prevents interaction with Ub in vitro and in cells. Crystallographic analysis shows that it blocks the ZnF pocket where Ub engages. Conditional expression of this DARPin reversibly impairs infection by IAV and Zika virus; moreover, SGs and aggresomes are downregulated. These results validate the HDAC6 ZnF as an attractive target for drug discovery.
- Friedrich Miescher Institute for Biomedical Research, Maulbeerstrasse 66, 4058 Basel, Switzerland; Faculty of Sciences, University of Basel, 4031 Basel, Switzerland.
Organizational Affiliation: