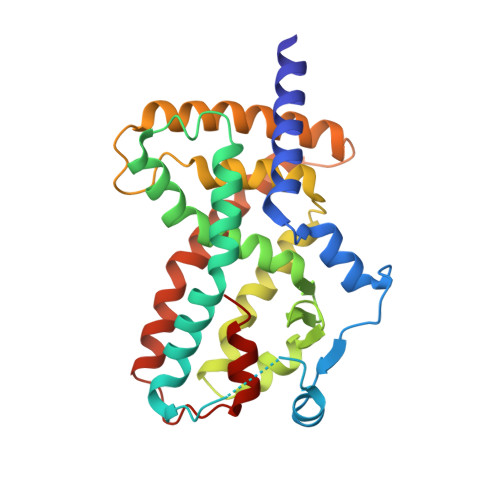
Biochemical and structural basis for the pharmacological inhibition of nuclear hormone receptor PPAR gamma by inverse agonists.
Irwin, S., Karr, C., Furman, C., Tsai, J., Gee, P., Banka, D., Wibowo, A.S., Dementiev, A.A., O'Shea, M., Yang, J., Lowe, J., Mitchell, L., Ruppel, S., Fekkes, P., Zhu, P., Korpal, M., Larsen, N.A.(2022) J Biol Chem 298: 102539-102539
- PubMed: 36179791
- DOI: https://doi.org/10.1016/j.jbc.2022.102539
- Primary Citation of Related Structures:
8DKN, 8DKV, 8DSY, 8DSZ - PubMed Abstract:
Recent studies have reported that the peroxisome proliferator-activated receptor gamma (PPARγ) pathway is activated in approximately 40% of patients with muscle-invasive bladder cancer. This led us to investigate pharmacological repression of PPARγ as a possible intervention strategy. Here, we characterize PPARγ antagonists and inverse agonists and find that the former behave as silent ligands, whereas inverse agonists (T0070907 and SR10221) repress downstream PPARγ target genes leading to growth inhibition in bladder cancer cell lines. To understand the mechanism, we determined the ternary crystal structure of PPARγ bound to T0070907 and the corepressor (co-R) peptide NCOR1. The structure shows that the AF-2 helix 12 (H12) rearranges to bind inside the ligand-binding domain, where it forms stabilizing interactions with the compound. This dramatic movement in H12 unveils a large interface for co-R binding. In contrast, the crystal structure of PPARγ bound to a SR10221 analog shows more subtle structural differences, where the compound binds and pushes H12 away from the ligand-binding domain to allow co-R binding. Interestingly, we found that both classes of compound promote recruitment of co-R proteins in biochemical assays but with distinct conformational changes in H12. We validate our structural models using both site-directed mutagenesis and chemical probes. Our findings offer new mechanistic insights into pharmacological modulation of PPARγ signaling.
Organizational Affiliation:
Department of Drug Discovery, H3 Biomedicine, Cambridge, Massachusetts, USA.