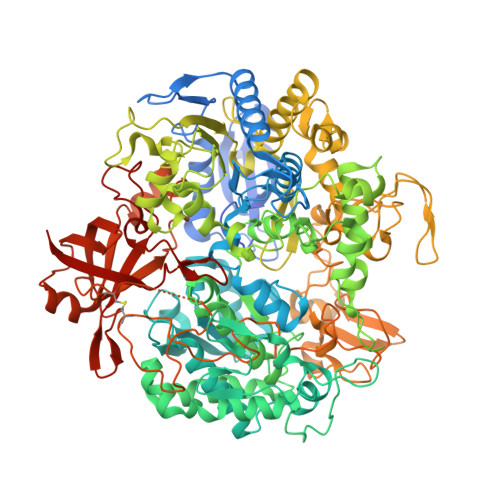
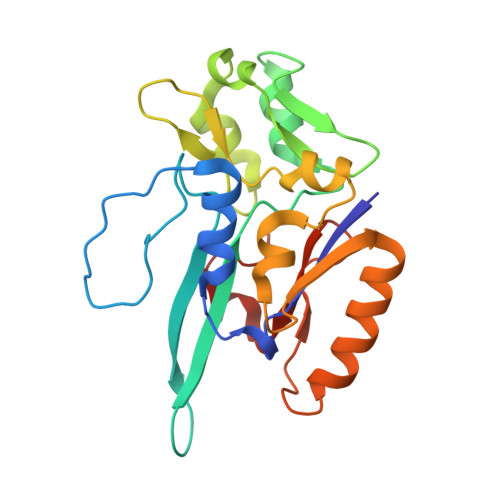
Structural and biochemical characterization of the M405S variant of Desulfovibrio vulgaris formate dehydrogenase.
Vilela-Alves, G., Rebelo Manuel, R., Pedrosa, N., Cardoso Pereira, I.A., Romao, M.J., Mota, C.(2024) Acta Crystallogr F Struct Biol Commun 80: 98-106
- PubMed: 38699971
- DOI: https://doi.org/10.1107/S2053230X24003911
- Primary Citation of Related Structures:
8RCG - PubMed Abstract:
Molybdenum- or tungsten-dependent formate dehydrogenases have emerged as significant catalysts for the chemical reduction of CO 2 to formate, with biotechnological applications envisaged in climate-change mitigation. The role of Met405 in the active site of Desulfovibrio vulgaris formate dehydrogenase AB (DvFdhAB) has remained elusive. However, its proximity to the metal site and the conformational change that it undergoes between the resting and active forms suggests a functional role. In this work, the M405S variant was engineered, which allowed the active-site geometry in the absence of methionine S δ interactions with the metal site to be revealed and the role of Met405 in catalysis to be probed. This variant displayed reduced activity in both formate oxidation and CO 2 reduction, together with an increased sensitivity to oxygen inactivation.
- UCIBIO, Applied Molecular Biosciences Unit, Department of Chemistry, NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal.
Organizational Affiliation: