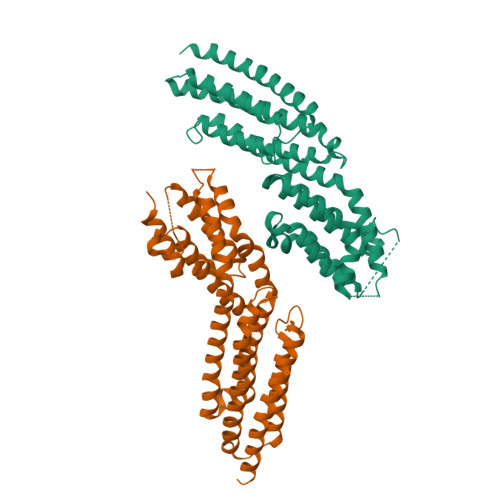
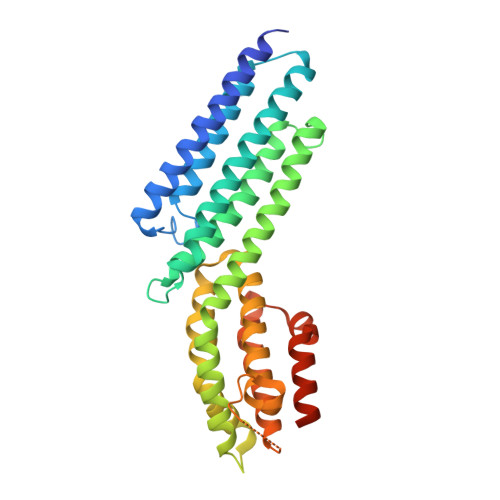
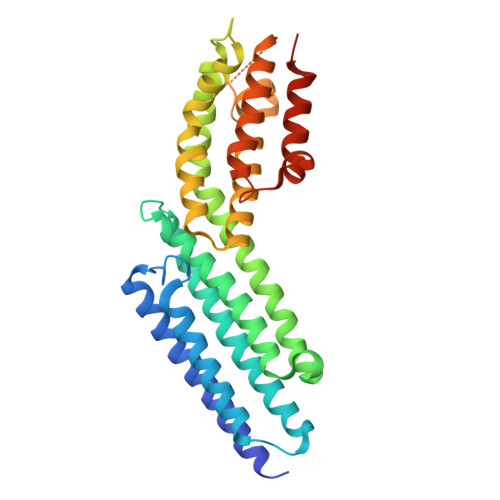
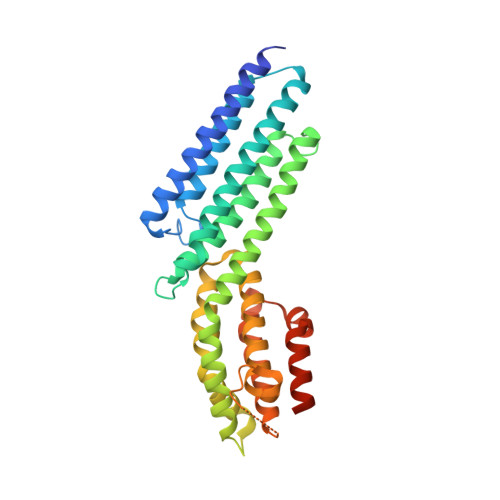
The Crystal Structure of a Munc13 C-terminal Module Exhibits a Remarkable Similarity to Vesicle Tethering Factors.
Li, W., Ma, C., Guan, R., Xu, Y., Tomchick, D.R., Rizo, J.(2011) Structure 19: 1443-1455
- PubMed: 22000513
- DOI: https://doi.org/10.1016/j.str.2011.07.012
- Primary Citation of Related Structures:
3SWH - PubMed Abstract:
Unc13/Munc13s play a crucial function in neurotransmitter release through their MUN domain, which mediates the transition from the Syntaxin-1/Munc18-1 complex to the SNARE complex. The MUN domain was suggested to be related to tethering factors, but no MUN-domain structure is available to experimentally validate this notion and address key unresolved questions about the interactions and minimal structural unit required for Unc13/Munc13 function. Here we identify an autonomously folded module within the MUN domain (MUN-CD) and show that its crystal structure is remarkably similar to several tethering factors. We also show that the activity in promoting the Syntaxin-1/Munc18-1 to SNARE complex transition is strongly impaired in MUN-CD. These results show that MUN domains and tethering factors indeed belong to the same family and may have a common role in membrane trafficking. We propose a model whereby the MUN-CD module is central for Munc13 function but full activity requires adjacent sequences.
Organizational Affiliation:
Department of Biochemistry, University of Texas Southwestern Medical Center, 6000 Harry Hines Boulevard, Dallas, TX 75390, USA.