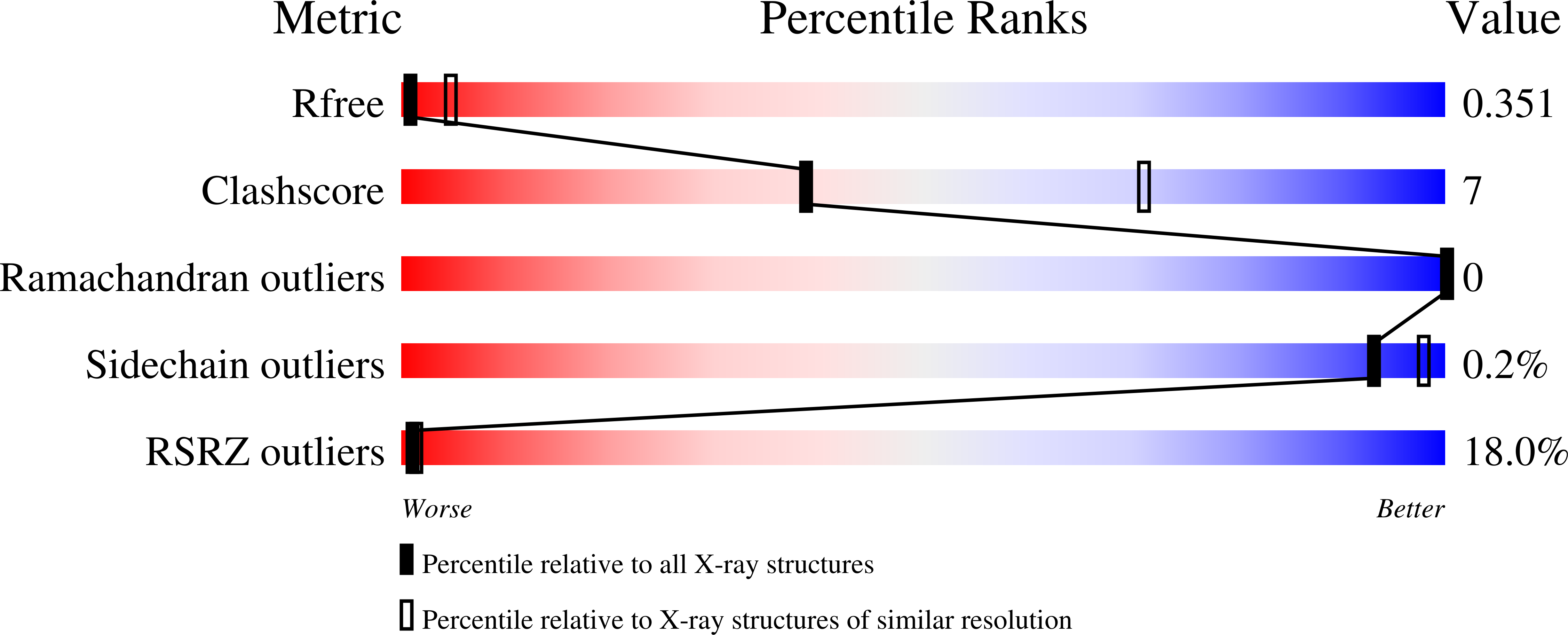
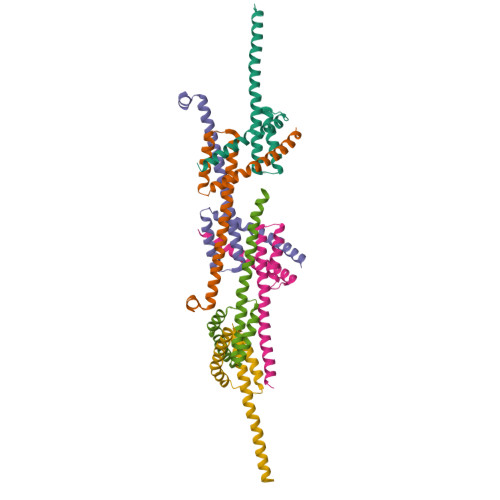
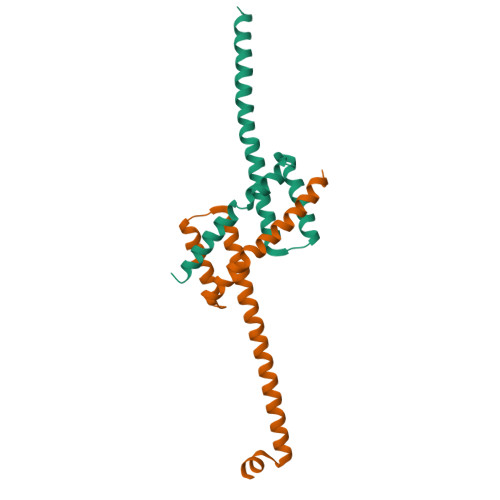
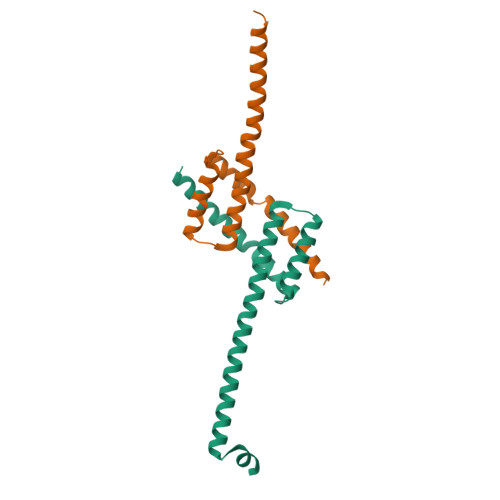
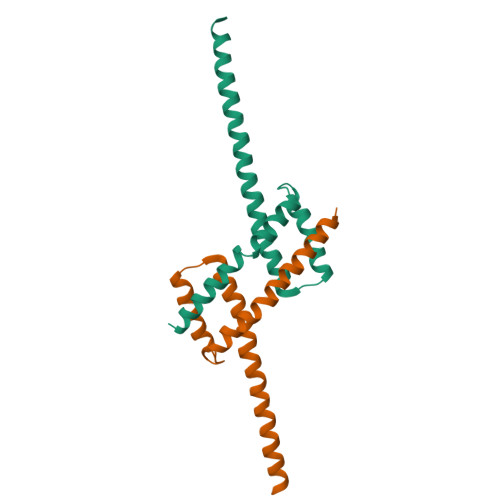
Structure of detergent-activated BAK dimers derived from the inert monomer.
Birkinshaw, R.W., Iyer, S., Lio, D., Luo, C.S., Brouwer, J.M., Miller, M.S., Robin, A.Y., Uren, R.T., Dewson, G., Kluck, R.M., Colman, P.M., Czabotar, P.E.(2021) Mol Cell 81: 2123
- PubMed: 33794146
- DOI: https://doi.org/10.1016/j.molcel.2021.03.014
- Primary Citation of Related Structures:
7K02 - PubMed Abstract:
A body of data supports the existence of core (α2-α5) dimers of BAK and BAX in the oligomeric, membrane-perturbing conformation of these essential apoptotic effector molecules. Molecular structures for these dimers have only been captured for truncated constructs encompassing the core domain alone. Here, we report a crystal structure of BAK α2-α8 dimers (i.e., minus its flexible N-terminal helix and membrane-anchoring C-terminal segment) that has been obtained through the activation of monomeric BAK with the detergent C12E8. Core dimers are evident, linked through the crystal by contacts via latch (α6-α8) domains. This crystal structure shows activated BAK dimers with the extended latch domain present. Our data provide direct evidence for the conformational change converting BAK from inert monomer to the functional dimer that destroys mitochondrial integrity. This dimer is the smallest functional unit for recombinant BAK or BAX described so far.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Melbourne, VIC 3052, Australia; Department of Medical Biology, University of Melbourne, Melbourne, VIC 3010, Australia. Electronic address: birkinshaw.r@wehi.edu.au.