Structural basis underlying the synergism of NADase and SLO during group A Streptococcus infection.
Tsai, W.J., Lai, Y.H., Shi, Y.A., Hammel, M., Duff, A.P., Whitten, A.E., Wilde, K.L., Wu, C.M., Knott, R., Jeng, U.S., Kang, C.Y., Hsu, C.Y., Wu, J.L., Tsai, P.J., Chiang-Ni, C., Wu, J.J., Lin, Y.S., Liu, C.C., Senda, T., Wang, S.(2023) Commun Biol 6: 124-124
- PubMed: 36721030
- DOI: https://doi.org/10.1038/s42003-023-04502-0
- Primary Citation of Related Structures:
7WVH - PubMed Abstract:
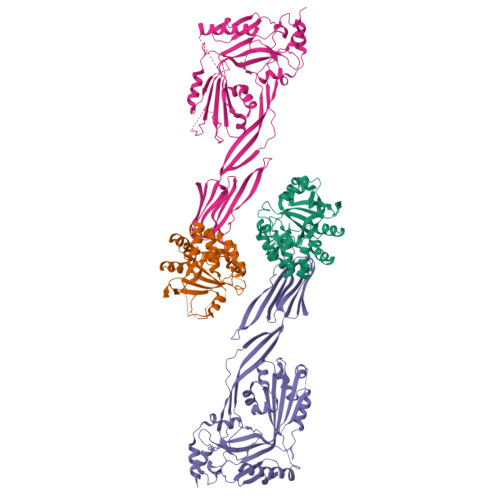
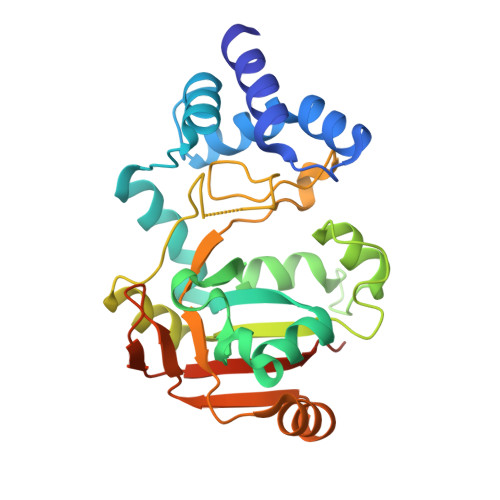
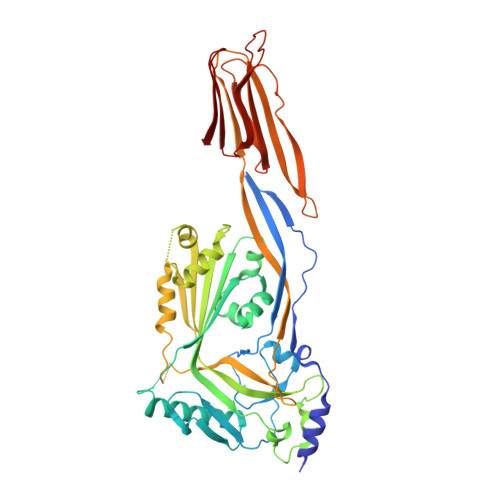
Group A Streptococcus (GAS) is a strict human pathogen possessing a unique pathogenic trait that utilizes the cooperative activity of NAD + -glycohydrolase (NADase) and Streptolysin O (SLO) to enhance its virulence. How NADase interacts with SLO to synergistically promote GAS cytotoxicity and intracellular survival is a long-standing question. Here, the structure and dynamic nature of the NADase/SLO complex are elucidated by X-ray crystallography and small-angle scattering, illustrating atomic details of the complex interface and functionally relevant conformations. Structure-guided studies reveal a salt-bridge interaction between NADase and SLO is important to cytotoxicity and resistance to phagocytic killing during GAS infection. Furthermore, the biological significance of the NADase/SLO complex in GAS virulence is demonstrated in a murine infection model. Overall, this work delivers the structure-functional relationship of the NADase/SLO complex and pinpoints the key interacting residues that are central to the coordinated actions of NADase and SLO in the pathogenesis of GAS infection.
Organizational Affiliation:
Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan.