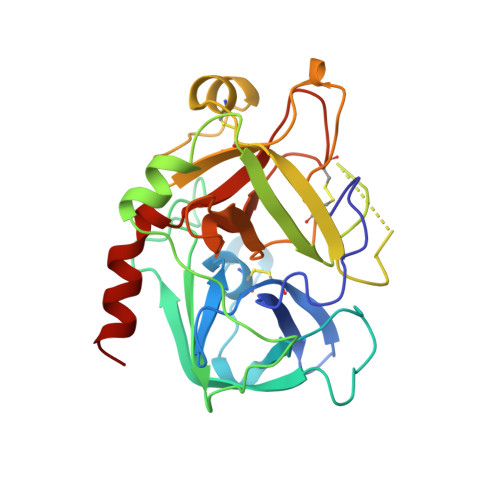
Molecular design and characterization of an alpha-thrombin inhibitor containing a novel P1 moiety.
Malikayil, J.A., Burkhart, J.P., Schreuder, H.A., Broersma Jr., R.J., Tardif, C., Kutcher 3rd., L.W., Mehdi, S., Schatzman, G.L., Neises, B., Peet, N.P.(1997) Biochemistry 36: 1034-1040
- PubMed: 9033393
- DOI: https://doi.org/10.1021/bi9622231
- Primary Citation of Related Structures:
1AD8 - PubMed Abstract:
An inhibitor of alpha-thrombin was designed on the basis of the X-ray crystal structures of thrombin and trypsin. The design strategy employed the geometric and electrostatic differences between the specificity pockets of the two enzymes. These differences arise due to the replacement of Ser 190 in trypsin by Ala 190 in thrombin. The new inhibitor contained a tryptophan side chain instead of the arginine side chain that is present in the prototypical thrombin inhibitors. This inhibitor had a Ki value of 0.25 microM, displayed more than 400-fold specificity for thrombin over trypsin, and doubled the rat plasma APTT at a concentration of 44.9 microM. The X-ray crystal structure of the inhibitor/alpha-thrombin complex was determined. This represents the first reported three-dimensional structure of a thrombin/ inhibitor complex where the specificity pocket of the enzyme is occupied by a chemical moiety other than a guanidino or an amidino group. As was predicted by the molecular model, the tryptophan side chain docks into the specificity pocket of the enzyme. This finding is in contrast with the indole binding region of thrombin reported earlier [Berliner, L. J., & Shen, Y. Y. L. (1977) Biochemistry 16, 4622-4626]. The lower binding affinity of the new inhibitor for trypsin, compared to that for thrombin, appears to be due to (i) the extra energy required to deform the smaller specificity pocket of trypsin to accommodate the bulky indole group and (ii) the favorable electrostatic interactions of the indole group with the more hydrophobic specificity pocket of thrombin. The neutral indole group may be of pharmacological significance because the severe hypotension and respiratory distress observed following the administration of some thrombin inhibitors have been linked to the positively charged guanidino or amidino functionalities.
- Hoechst Marion Roussel, Inc., Cincinnati, Ohio 45215, USA.
Organizational Affiliation: