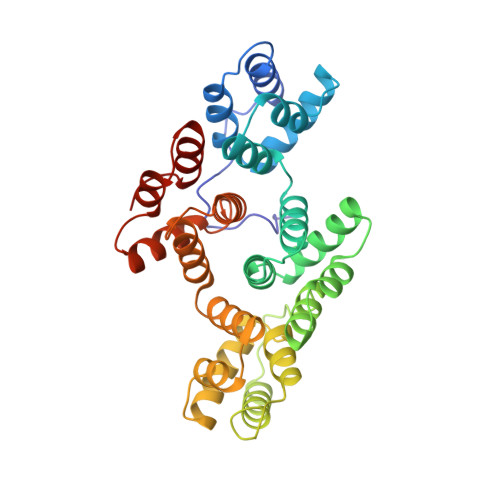
The high-resolution crystal structure of human annexin III shows subtle differences with annexin V.
Favier-Perron, B., Lewit-Bentley, A., Russo-Marie, F.(1996) Biochemistry 35: 1740-1744
- PubMed: 8639653
- DOI: https://doi.org/10.1021/bi952092o
- Primary Citation of Related Structures:
1AXN - PubMed Abstract:
The structure of recombinant human annexin III was solved to 1.8 A resolution. Though homologous to annexin I and V, the annexin III structure shows significant differences. The tryptophan in the calcium loop of the third domain is exposed to the solvent, as in the structure of annexin V crystallized in high calcium concentrations, although the annexin III crystals were prepared at low calcium concentrations. The position of domain III relative to the other domains is different from both annexin V and I, suggesting further flexibility of the molecule. The entire N-terminus of the protein is well-defined in the present structure. The side chain of tryptophan 5 interacts with the hinge region of the hydrophillic channel, which could have an effect on the potential mobility of this region, as well as on its possible calcium channel behavior.
- ICGM, U332 INSERM, Paris, France.
Organizational Affiliation: