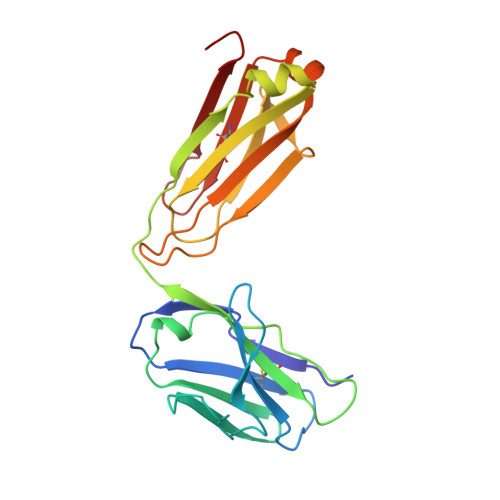
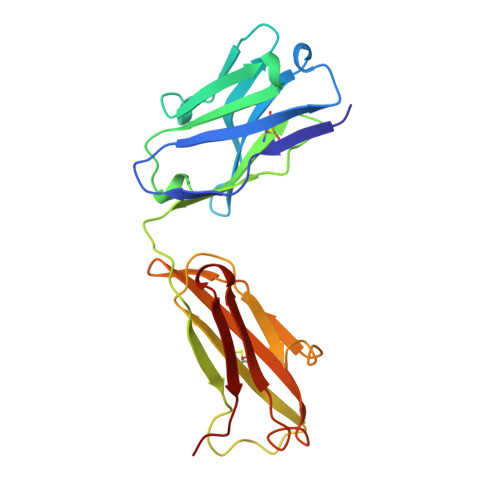
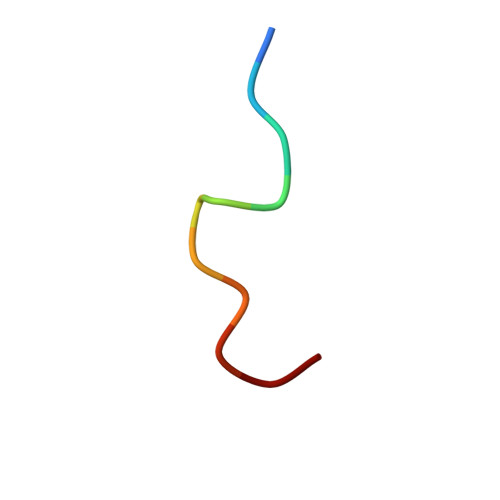
Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross-reactivity and polyspecificity.
Keitel, T., Kramer, A., Wessner, H., Scholz, C., Schneider-Mergener, J., Hohne, W.(1997) Cell 91: 811-820
- PubMed: 9413990
- DOI: https://doi.org/10.1016/s0092-8674(00)80469-9
- Primary Citation of Related Structures:
1BOG, 1CFN, 1CFQ, 1CFS, 1CFT, 1HI6 - PubMed Abstract:
The X-ray crystal structures of an anti-p24 (HIV-1) monoclonal antibody Fab fragment alone and in complexes with the epitope peptide GATPQDLNTnL (n = norleucine), an epitope-homologous peptide GATPEDLNQKLAGN, as well as two unrelated peptides GLYEWGGARITNTD and efslkGpllqwrsG (D-peptide), are presented to a maximum resolution of 2.6 A. The latter three peptides were identified from screening synthetic combinatorial peptide libraries. Although all peptides bind to the same antigen combining site, the nonhomologous peptides adopt different binding conformations and also form their critical contacts with different antibody residues. Only small readjustments are observed within the framework of the Fab fragment upon binding.
- Institut für Biochemie, Universitätsklinikum Charité, Humboldt-Universität zu Berlin, Germany.
Organizational Affiliation: