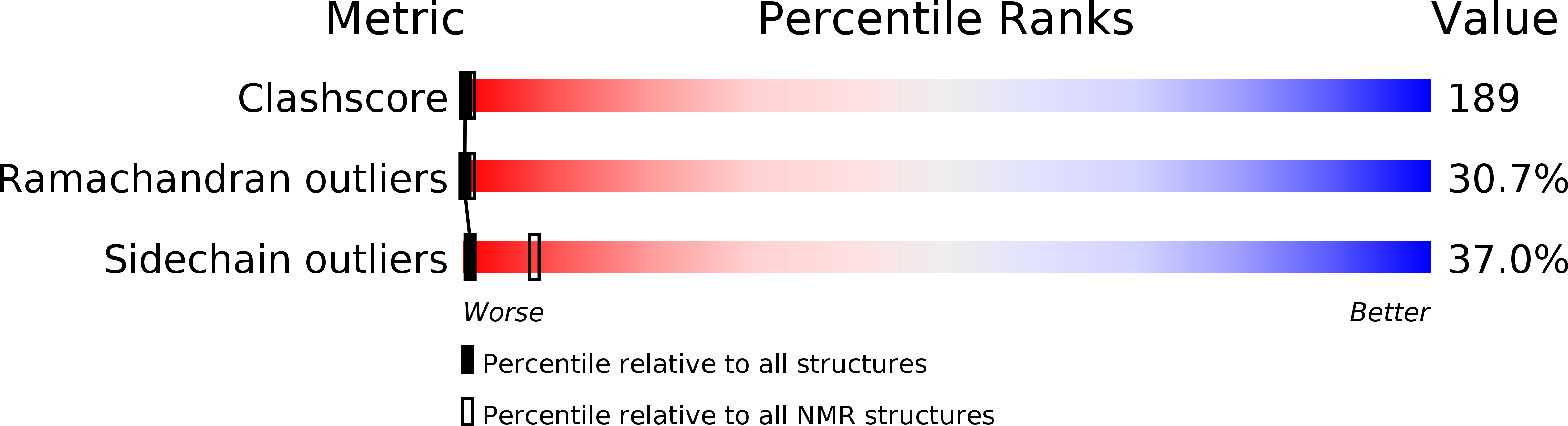1H and 15N NMR sequential assignment, secondary structure, and tertiary fold of [2Fe-2S] ferredoxin from Synechocystis sp. PCC 6803.
Lelong, C., Setif, P., Bottin, H., Andre, F., Neumann, J.M.(1995) Biochemistry 34: 14462-14473
- PubMed: 7578051
- DOI: https://doi.org/10.1021/bi00044a024
- Primary Citation of Related Structures:
1DOX, 1DOY - PubMed Abstract:
The [2Fe-2S] ferredoxin extracted from Synechocystis sp. PCC 6803 was studied by 1H and 15N nuclear magnetic resonance. Sequence-specific 1H and 15N assignment of amino acid residues far from the paramagnetic cluster (distance higher than 8 A) was performed. Interresidue NOE constraints have allowed the identification of several secondary structure elements: one beta sheet composed of four beta strands, one alpha helix, and two alpha helix turns. The analysis of interresidue NOEs suggests the existence of a disulfide bridge between the cysteine residues 18 and 85. Such a disulfide bridge has never been observed in plant-type ferredoxins. Structure modeling using the X-PLOR program was performed with or without assuming the existence of a disulfide bridge. As a result, two structure families were obtained with rms deviations of 2.2 A. Due to the lack of NOE connectivities resulting from the paramagnetic effect from the [2Fe-2S] cluster, the structures were not well resolved in the region surrounding the [2Fe-2S] cluster, at both extremities of the alpha helix and the C and N terminus segments. In contrast, when taken separately, the beta sheet and the alpha helix were well defined. This work is the first report of a structure model of a plant-type [2Fe-2S] Fd in solution.
Organizational Affiliation:
CEA, Département de Biologie Cellulaire et Moléculaire, Gif sur Yvette, France.