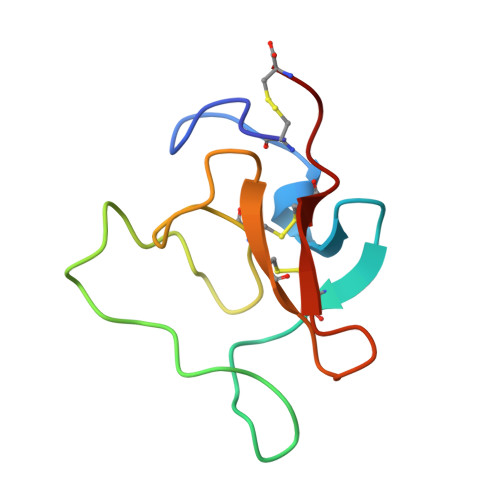
Solution structure of the epsilon-aminohexanoic acid complex of human plasminogen kringle 1.
Rejante, M.R., Llinas, M.(1994) Eur J Biochem 221: 939-949
- PubMed: 8181476
- DOI: https://doi.org/10.1111/j.1432-1033.1994.tb18809.x
- Primary Citation of Related Structures:
1HPJ, 1HPK - PubMed Abstract:
The solution structure of the human plasminogen kringle 1 domain complexed to the antifibrinolytic drug 6-aminohexanoic acid (epsilon Ahx) was obtained on the basis of 1H-NMR spectroscopic data and dynamical simulated annealing calculations. Two sets of structures were derived starting from (a) random coil conformations and (b) the (mutated) crystallographic structure of the homologous prothrombin kringle 1. The two sets display essentially the same backbone folding (pairwise root-mean-square deviation, 0.15 nm) indicating that, regardless of the initial structure, the data is sufficient to locate a conformation corresponding to an essentially unique energy minimum. The conformations of residues connected to prolines were localized to energetically preferred regions of the Ramachandran map. The Pro30 peptide bond is proposed to be cis. The ligand-binding site of the kringle 1 is a shallow cavity composed of Pro33, Phe36, Trp62, Tyr64, Tyr72 and Tyr74. Doubly charged anionic and cationic centers configured by the side chains of Asp55 and Asp57, and Arg34 and Arg71, respectively, contribute to anchoring the zwitterionic epsilon Ahx molecule at the binding site. The ligand exhibits closer contacts with the kringle anionic centers (approximately 0.35 nm average O...H distance between the Asp55/Asp57 carboxylate and ligand amino groups) than with the cationic ones (approximately 0.52 nm closest O...H distances between the ligand carboxylate and the Arg34/Arg71 guanidino groups). The epsilon Ahx hydrocarbon chain rests flanked by Pro33, Tyr64, Tyr72 and Tyr74 on one side and Phe36 on the other. Dipolar (Overhauser) connectivities indicate that the ligand aliphatic moiety establishes close contacts with the Phe36 and Trp62 aromatic rings. The computed structure suggests that the epsilon Ahx molecule adopts a kinked conformation when complexed to kringle 1, effectively shortening its dipole length to approximately 0.65 nm.
- Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213-3890.
Organizational Affiliation: