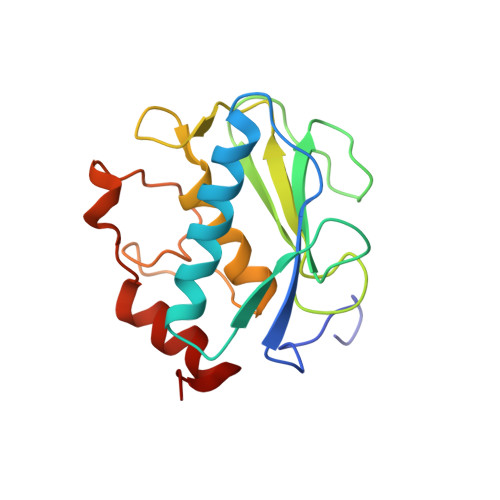
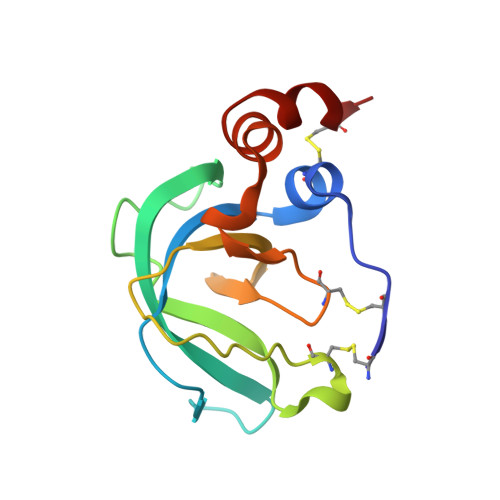
Global Orientation of Bound MMP-3 and N-TIMP-1 in Solution via Residual Dipolar Couplings
Arumugam, S., Van Doren, S.R.(2003) Biochemistry 42: 7950-7958
- PubMed: 12834347
- DOI: https://doi.org/10.1021/bi034545s
- Primary Citation of Related Structures:
1OO9 - PubMed Abstract:
Crystal structures of catalytic domains of MMP-3 and MT1-MMP bound to TIMP-1 or TIMP-2, respectively, differ in the orientation of the TIMP in the MMP active site. The orientation in solution of N-TIMP-1 in the MMP-3 active site has been investigated using residual dipolar couplings (RDCs). Fitting of the RDCs to the X-ray structures of the complexes suggests general agreement with the orientation of crystalline MMP-3(DeltaC) and TIMP-1 and a large disparity from the orientation of crystalline MT1-MMP(DeltaC) and TIMP-2. Rigid body docking of MMP-3 and N-TIMP-1 X-ray coordinates using RDCs and intermolecular NOEs provided a time-averaged orientation in solution differing from the crystal structure by a 5 degrees rotation toward the MT1-MMP(DeltaC)/TIMP-2 orientation. The slight discrepancy in orientations in solution and crystal lies within the experimental uncertainties. Intermolecular NOEs used in the docking corroborated the accuracy of mapping the interface by a paramagnetic NMR footprinting assay, a potential alternative source of contacts for docking. Some uncertainty in the N-TIMP-1 orientation in the MMP-3 active site, coupled with microsecond to millisecond fluctuations of the MMP-binding ridge of N-TIMP-1 in the complex and flexibility in MMP-3(DeltaC) S(1)' to S(3)' subsites, leaves open the possibility that N-TIMP-1 might dynamically pivot a few degrees or more in the arc toward the MT1-MMP(DeltaC)/TIMP-2 orientation. Differing TIMP orientations in MMP active sites are more likely to result from structural differences in TIMP AB hairpin loops than from crystal packing artifacts.
- Department of Biochemistry, 117 Schweitzer Hall, University of Missouri, Columbia, Missouri 65211, USA.
Organizational Affiliation: