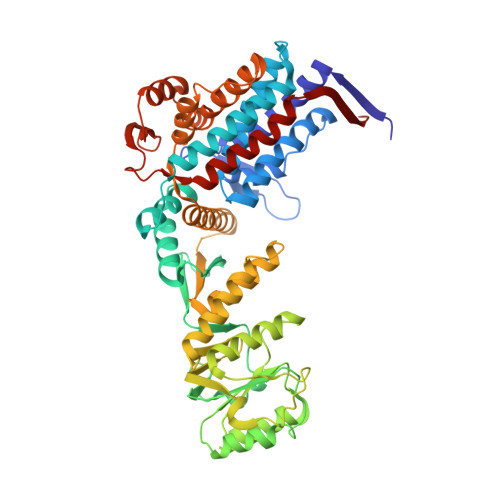
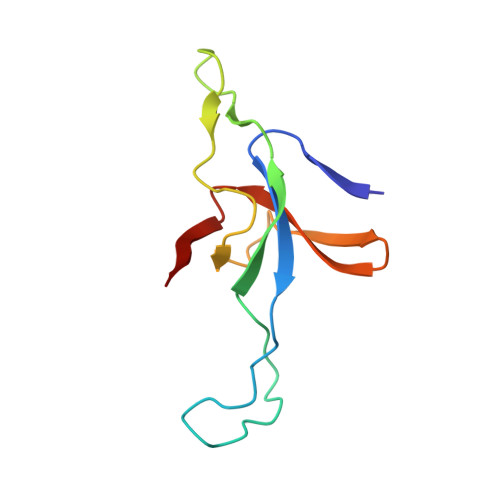
Role of the gamma-phosphate of ATP in triggering protein folding by GroEL-GroES: function, structure and energetics.
Chaudhry, C., Farr, G.W., Todd, M.J., Rye, H.S., Brunger, A.T., Adams, P.D., Horwich, A.L., Sigler, P.B.(2003) EMBO J 22: 4877-4887
- PubMed: 14517228
- DOI: https://doi.org/10.1093/emboj/cdg477
- Primary Citation of Related Structures:
1PCQ, 1PF9 - PubMed Abstract:
Productive cis folding by the chaperonin GroEL is triggered by the binding of ATP but not ADP, along with cochaperonin GroES, to the same ring as non-native polypeptide, ejecting polypeptide into an encapsulated hydrophilic chamber. We examined the specific contribution of the gamma-phosphate of ATP to this activation process using complexes of ADP and aluminium or beryllium fluoride. These ATP analogues supported productive cis folding of the substrate protein, rhodanese, even when added to already-formed, folding-inactive cis ADP ternary complexes, essentially introducing the gamma-phosphate of ATP in an independent step. Aluminium fluoride was observed to stabilize the association of GroES with GroEL, with a substantial release of free energy (-46 kcal/mol). To understand the basis of such activation and stabilization, a crystal structure of GroEL-GroES-ADP.AlF3 was determined at 2.8 A. A trigonal AlF3 metal complex was observed in the gamma-phosphate position of the nucleotide pocket of the cis ring. Surprisingly, when this structure was compared with that of the previously determined GroEL-GroES-ADP complex, no other differences were observed. We discuss the likely basis of the ability of gamma-phosphate binding to convert preformed GroEL-GroES-ADP-polypeptide complexes into the folding-active state.
- Department of Molecular Biophysics and Biochemistry and Howard Hughes Medical Institute, Yale University, New Haven, CT, USA.
Organizational Affiliation: