Two crystal structures of the B1 immunoglobulin-binding domain of streptococcal protein G and comparison with NMR.
Gallagher, T., Alexander, P., Bryan, P., Gilliland, G.L.(1994) Biochemistry 33: 4721-4729
- PubMed: 8161530
- Primary Citation of Related Structures:
1PGA, 1PGB - PubMed Abstract:
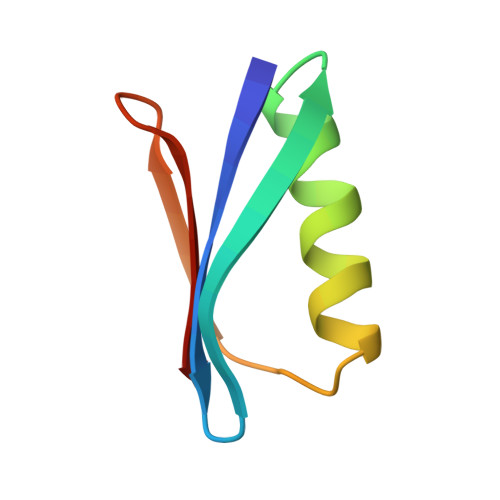
The structure of the 56-residue B1 immunoglobulin-binding domain from streptococcal protein G has been determined in two different crystal forms. The crystal structures were deduced by molecular replacement, based on the structure of the B2 domain (Brookhaven accession code 1PGX). Final R values are 0.174 and 0.198 for orthorhombic and trigonal forms, for diffraction data from 6.0 to 2.07 A and from 6 to 1.92 A, respectively. The orthorhombic crystals have an unusually high packing density for protein crystals, with Vm = 1.66 and a solvent content of 26%. The protein structure is found to be very similar (rms deviation 0.25 A for 56 C alpha's) in the two crystal forms, with an efficiently packed hydrophobic core between a four-stranded beta-sheet and a four-turn alpha-helix. The B1 domain has the same fold and general structure as the B2 domain (rms deviations 0.36 and 0.39 A), despite the six residue differences between them. The crystallographic models differ from NMR-derived models in several local regions, primarily in the loop involving residues 46-51; other significant variations are observed in the helix and in the structure of bound water. The primary crystal contact is the same in both crystal forms, involving both sheet edges to form extended beta-sheets throughout the crystals.
- Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute, Rockville 20850.
Organizational Affiliation: