JAMM: a metalloprotease-like zinc site in the proteasome and signalosome.
Ambroggio, X.I., Rees, D.C., Deshaies, R.J.(2004) PLoS Biol 2: E2-E2
- PubMed: 14737182
- DOI: https://doi.org/10.1371/journal.pbio.0020002
- Primary Citation of Related Structures:
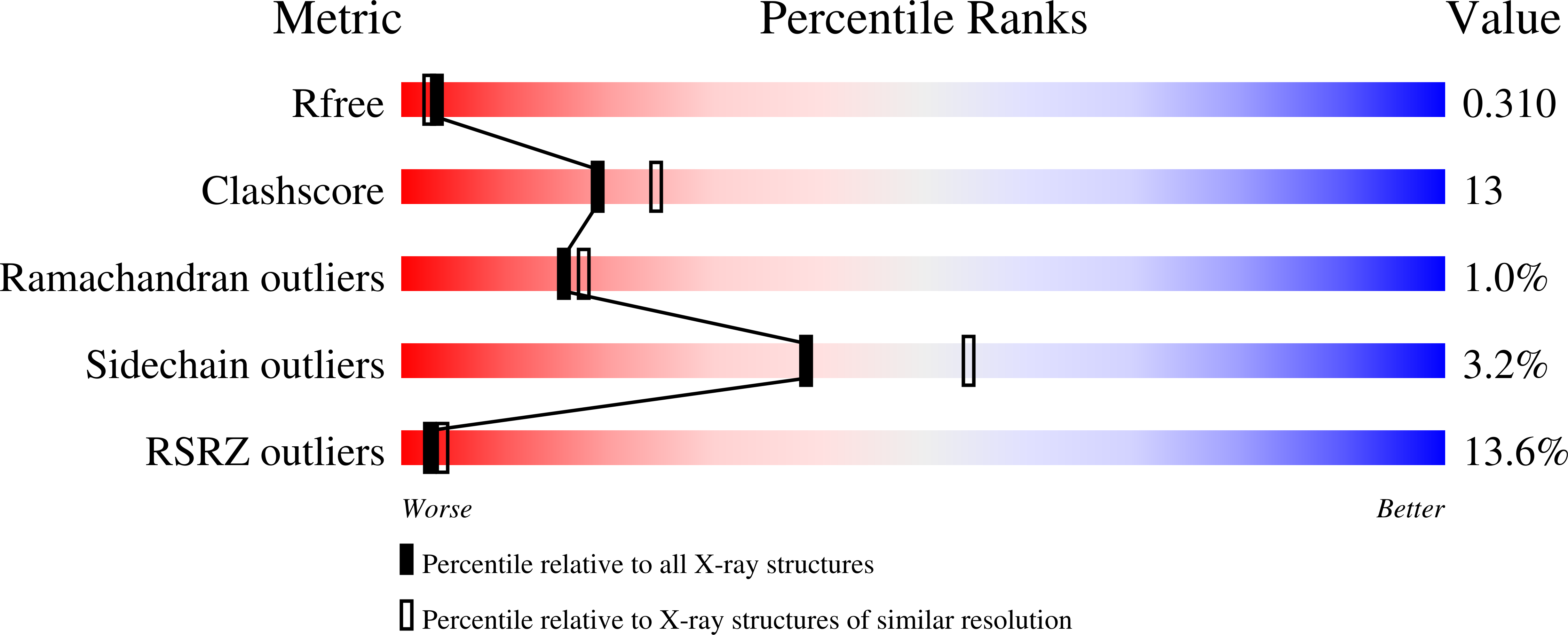
1R5X - PubMed Abstract:
The JAMM (JAB1/MPN/Mov34 metalloenzyme) motif in Rpn11 and Csn5 underlies isopeptidase activities intrinsic to the proteasome and signalosome, respectively. We show here that the archaebacterial protein AfJAMM possesses the key features of a zinc metalloprotease, yet with a distinct fold. The histidine and aspartic acid of the conserved EX(n)HS/THX(7)SXXD motif coordinate a zinc, whereas the glutamic acid hydrogen-bonds an aqua ligand. By analogy to the active site of thermolysin, we predict that the glutamic acid serves as an acid-base catalyst and the second serine stabilizes a tetrahedral intermediate. Mutagenesis of Csn5 confirms these residues are required for Nedd8 isopeptidase activity. The active site-like architecture specified by the JAMM motif motivates structure-based approaches to the study of JAMM domain proteins and the development of therapeutic proteasome and signalosome inhibitors.
Organizational Affiliation:
Division of Biology, California Institute of Technology, Pasadena, California, USA.