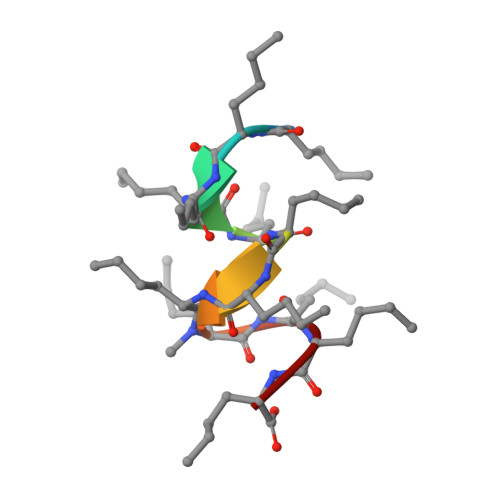
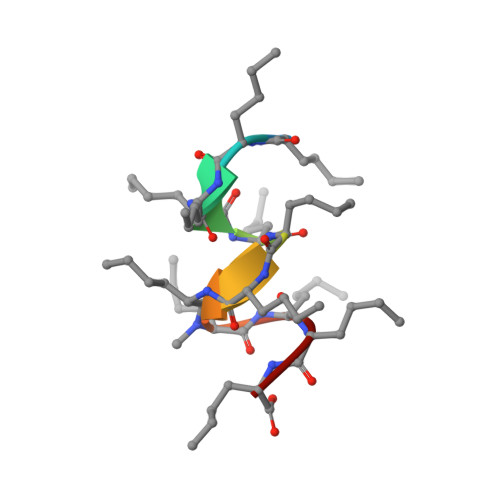
Solution NMR Structure of a D,L-Alternating Oligonorleucine as a Model of Beta-Helix
Navarro, E., Tejero, R., Fenude, E., Celda, B.(2001) Biopolymers 59: 110-119
- PubMed: 11373724
- DOI: https://doi.org/10.1002/1097-0282(200108)59:2<110::AID-BIP1010>3.0.CO;2-S
- Primary Citation of Related Structures:
1R9V - PubMed Abstract:
beta-Helix structures are of particular interest due to their capacity to form transmembrane channels with different transport properties. However, the relatively large number of beta-helices configurations does not allow a direct conformational analysis of beta-helical oligopeptides. A synthetic alternating D,L-oligopeptide with twelve norleucines (XIIMe) has been used as a model to get insight in the conformational features of beta-helix structures. The spatial configuration of XIIMe in solution has been determined by NMR. An extensive set of distances (nuclear Overhauser effect) and dihedral (J coupling constants) constraints have been included in molecular dynamics calculations. The NMR experimental data and theoretical calculations clearly indicate that the XIIMe adopts a single beta(4.4)-helix-type conformation in nonpolar solvents.
Organizational Affiliation:
Departamento de Química Física, Facultat de Químicas, Universitat de Valencia, C/Dr. Moliner, 50, 46100-Burjassot (Valencia), Spain.