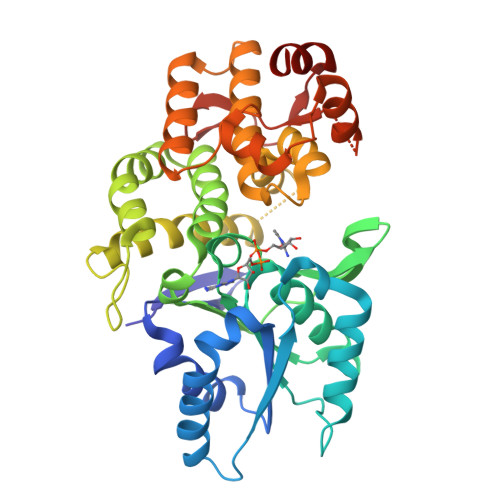
Structure of the 'open' form of Aspergillus nidulans 3-dehydroquinate synthase at 1.7 A resolution from crystals grown following enzyme turnover.
Nichols, C.E., Hawkins, A.R., Stammers, D.K.(2004) Acta Crystallogr D Biol Crystallogr 60: 971-973
- PubMed: 15103156
- DOI: https://doi.org/10.1107/S0907444904004743
- Primary Citation of Related Structures:
1SG6 - PubMed Abstract:
Crystallization of Aspergillus nidulans 3-dehydroquinate synthase (DHQS), following turnover of the enzyme by addition of the substrate DAHP, gave a new crystal form (form J). Although the crystals have dimensions of only 50 x 20 x 5 micro m, they are well ordered, diffracting to 1.7 A. The space group is C222(1), with unit-cell parameters a = 90.0, b = 103.7, c = 177.4 A. Structure determination and refinement to R = 0.19 (R(free) = 0.25) shows the DHQS is in the 'open' form with the substrate site unoccupied but with some loop regions perturbed. Previous crystals of open-form DHQS only diffracted to 2.5 A resolution. The use of enzyme turnover may be applicable in other systems in attempts to improve crystal quality.
Organizational Affiliation:
Division of Structural Biology, The Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, England.