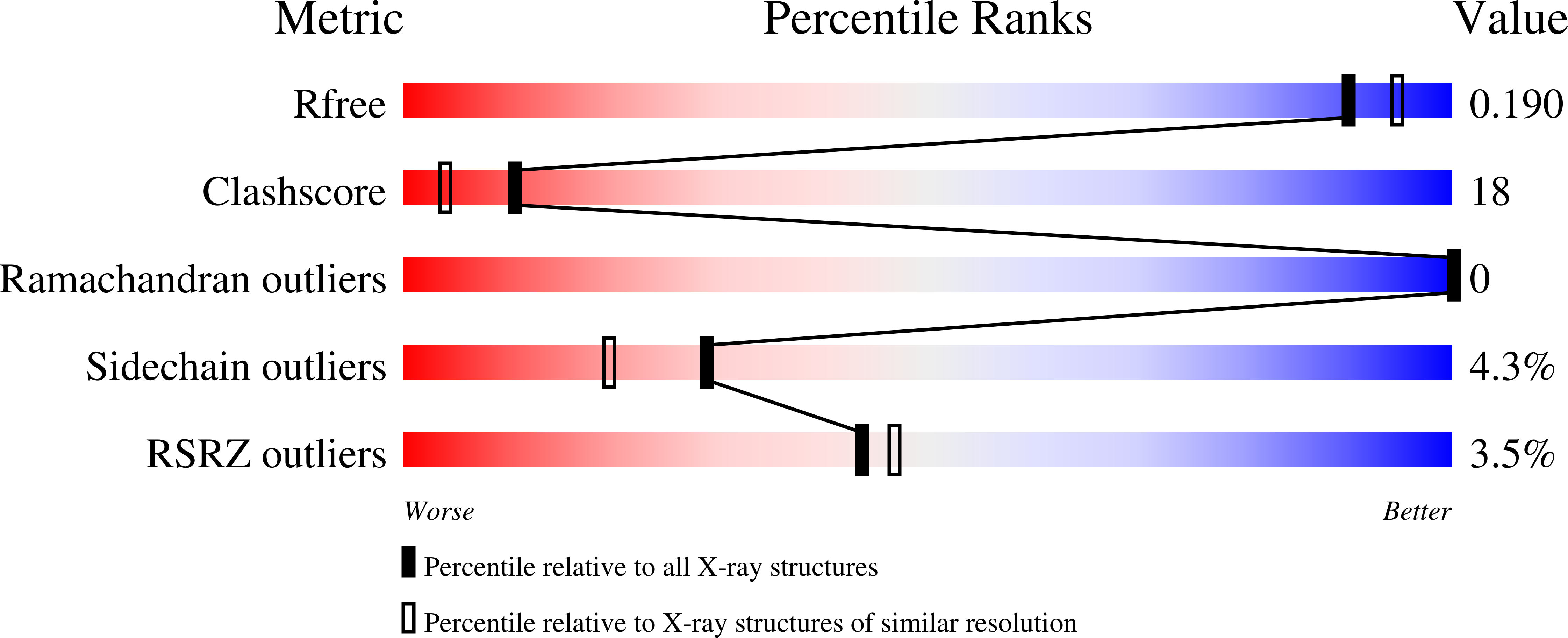
Secondary binding site of trypsin: revealed by crystal structure of trypsin-peptide complex.
Shamaladevi, N., Pattabhi, V.(2005) J Biomol Struct Dyn 22: 635-642
- PubMed: 15842169
- DOI: https://doi.org/10.1080/07391102.2005.10507031
- Primary Citation of Related Structures:
1V6D - PubMed Abstract:
Designed synthetic heterochiral peptides, when added to porcine trypsin, resulted in reduction of enzyme activity. The crystal structure of a complex formed between porcine trypsin and a heterochiral hepta peptide Boc-Pro-DAsp-Aib-Leu-Aib-Leu-Ala-NHMe has been determined at 1.9 A resolution. The hepta peptide does not bind at the active site, but is located in the interstitial region, and interacts with the calcium-binding loop (residues 60-80). The bound peptide interacts with the active site residue Ser195 through an acetate ion, and with Lys 60 mediated by water molecules. The structure, when compared with the other trypsin-peptide complexes, suggests that the flexibility of surface loops, concerted movement of the loops towards the active site, and the interaction of the bound peptide with Lys 60, may be responsible for the reduction in enzyme activity. This study provides a structural evidence for the earlier biochemical observation regarding the role of surface loops in the catalysis of the enzyme.
Organizational Affiliation:
Dept. of Crystallography and Biophysics, University of Madras, Guindy Campus, Chennai-600025, India.