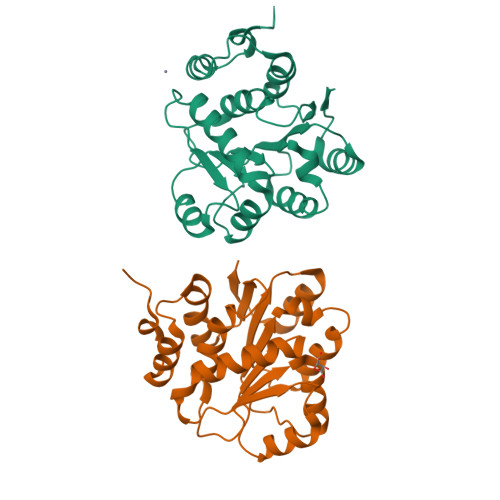
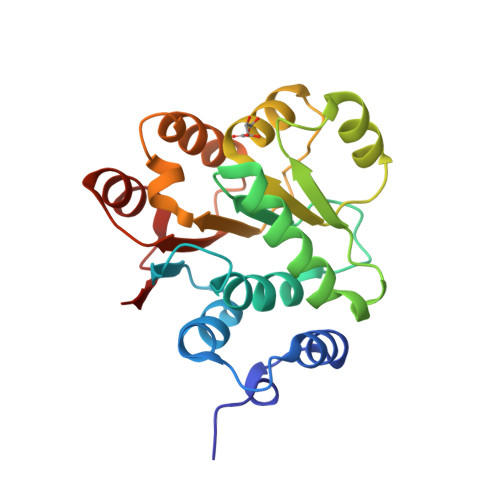
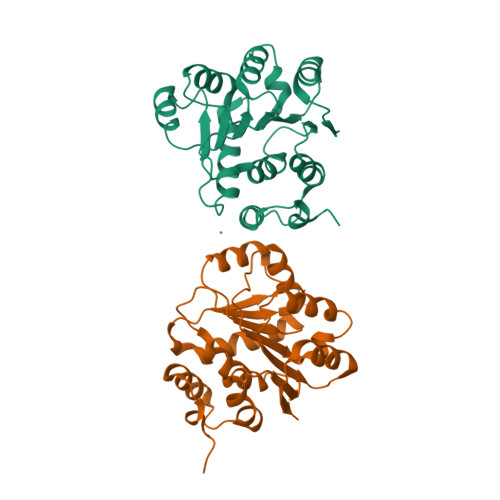
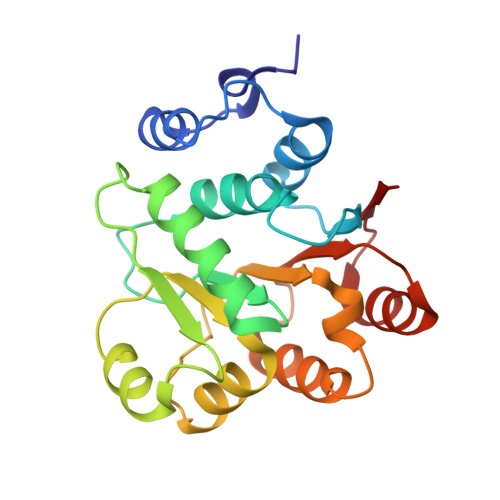
Structural insight of human DEAD-box protein rck/p54 into its substrate recognition with conformational changes
Matsui, T., Hogetsu, K., Usukura, J., Sato, T., Kumasaka, T., Akao, Y., Tanaka, N.(2006) Genes Cells 11: 439-452
- PubMed: 16611246
- DOI: https://doi.org/10.1111/j.1365-2443.2006.00951.x
- Primary Citation of Related Structures:
1VEC - PubMed Abstract:
Human rck/p54, a product of the gene cloned at the breakpoint of t(11; 14) (q23;q32) chromosomal translocation on 11q23 in B-cell lymphoma, is a member of the DEAD-box RNA helicase family. Here, the crystal structure of Nc-rck/p54, the N-terminal core domain of rck/p54, revealed that the P-loop in motif I formed a closed conformation, which was induced by Asn131, a residue unique to the RCK subfamily. It appears that ATP does not bind to the P-loop. The results of dynamic light scattering revealed to ATP-induced conformational change of rck/p54. It was demonstrated that free rck/p54 is a distended molecule in solution, and that the approach between N-terminal core and C-terminal domains for ATP binding would be essential when unwinding RNA. The results from helicase assay using electron micrograph, ATP hydrolytic and luciferase assay showed that c-myc IRES RNA, whose secondary structure regulates IRES-dependant translation, was unwound by rck/p54 and indicated that it is a good substrate for rck/p54. Over-expression of rck/p54 in HeLa cells caused growth inhibition and cell cycle arrest at G2/M with down-regulation of c-myc expression. These findings altogether suggest that rck/p54 may affect the IRES-dependent translation of c-myc even in the cells.
Organizational Affiliation:
Department of Life Science, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Nagatsuta-cho 4259, Midori-ku, Yokohama, 226-8501, Japan.