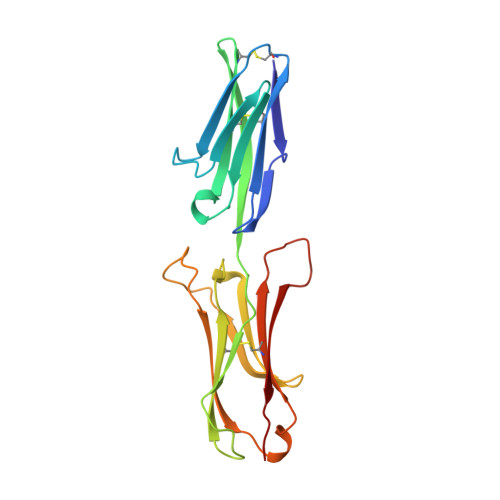
The crystal structure of an N-terminal two-domain fragment of vascular cell adhesion molecule 1 (VCAM-1): a cyclic peptide based on the domain 1 C-D loop can inhibit VCAM-1-alpha 4 integrin interaction.
Wang, J.H., Pepinsky, R.B., Stehle, T., Liu, J.H., Karpusas, M., Browning, B., Osborn, L.(1995) Proc Natl Acad Sci U S A 92: 5714-5718
- PubMed: 7539925
- DOI: https://doi.org/10.1073/pnas.92.12.5714
- Primary Citation of Related Structures:
1VSC - PubMed Abstract:
Vascular cell adhesion molecule 1 (VCAM-1) represents a structurally and functionally distinct class of immunoglobulin superfamily molecules that bind leukocyte integrins and are involved in inflammatory and immune functions. X-ray crystallography defines the three-dimensional structure of the N-terminal two-domain fragment that participates in ligand binding. Residues in domain 1 important for ligand binding reside in the C-D loop, which projects markedly from one face of the molecule near the contact between domains 1 and 2. A cyclic peptide that mimics this loop inhibits binding of alpha 4 beta 1 integrin-bearing cells to VCAM-1. These data demonstrate how crystallographic structural information can be used to design a small molecule inhibitor of biological function.
- Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138, USA.
Organizational Affiliation: