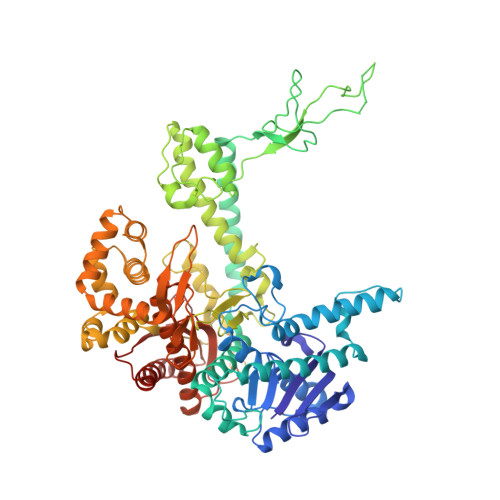
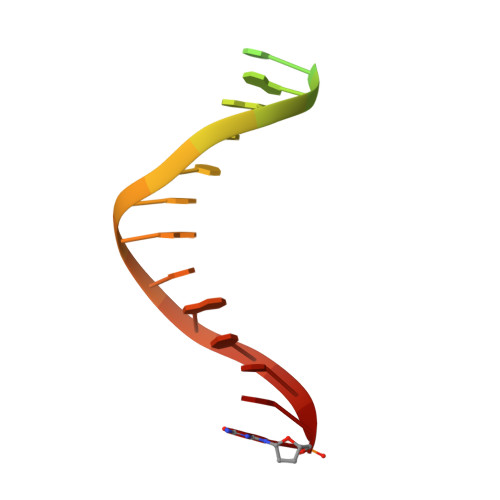
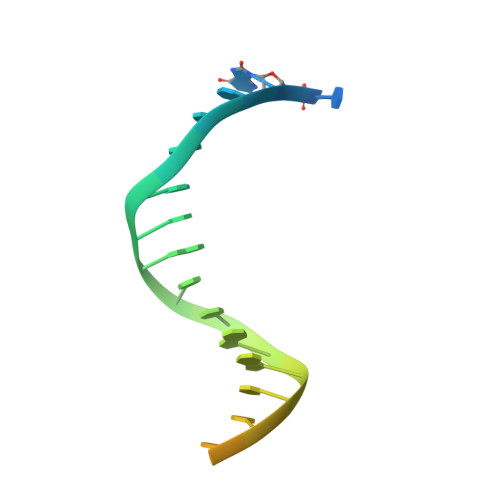
A lysine residue in the fingers subdomain of t7 DNA polymerase modulates the miscoding potential of 8-oxo-7,8-dihydroguanosine.
Brieba, L.G., Kokoska, R.J., Bebenek, K., Kunkel, T.A., Ellenberger, T.(2005) Structure 13: 1653-1659
- PubMed: 16271888
- DOI: https://doi.org/10.1016/j.str.2005.07.020
- Primary Citation of Related Structures:
1ZYQ - PubMed Abstract:
8-oxo-7,8-dihydroguanosine (8oG) is a highly mutagenic DNA lesion that stably pairs with adenosine, forming 8oG(syn).dA(anti) Hoogsteen base pairs. DNA polymerases show different propensities to insert dCMP or dAMP opposite 8oG, but the molecular mechanisms that determine faithful or mutagenic bypass are poorly understood. Here, we report kinetic and structural data providing evidence that, in T7 DNA polymerase, residue Lys536 is responsible for attenuating the miscoding potential of 8oG. The Lys536Ala polymerase shows a significant increase in mutagenic 8oG bypass versus wild-type polymerase, and a crystal structure of the Lys536Ala mutant reveals a closed complex with an 8oG(syn).dATP mismatch in the polymerase active site, in contrast to the unproductive, open complex previously obtained by using wild-type polymerase. We propose that Lys536 acts as a steric and/or electrostatic filter that attenuates the miscoding potential of 8oG by normally interfering with the binding of 8oG in a syn conformation that pairs with dATP.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Massachusetts 02115, USA.
Organizational Affiliation: