Crystal structure of the oligomerization domain of the phosphoprotein of vesicular stomatitis virus
Ding, H., Green, T.J., Lu, S., Luo, M.(2006) J Virol 80: 2808-2814
- PubMed: 16501089
- DOI: https://doi.org/10.1128/JVI.80.6.2808-2814.2006
- Primary Citation of Related Structures:
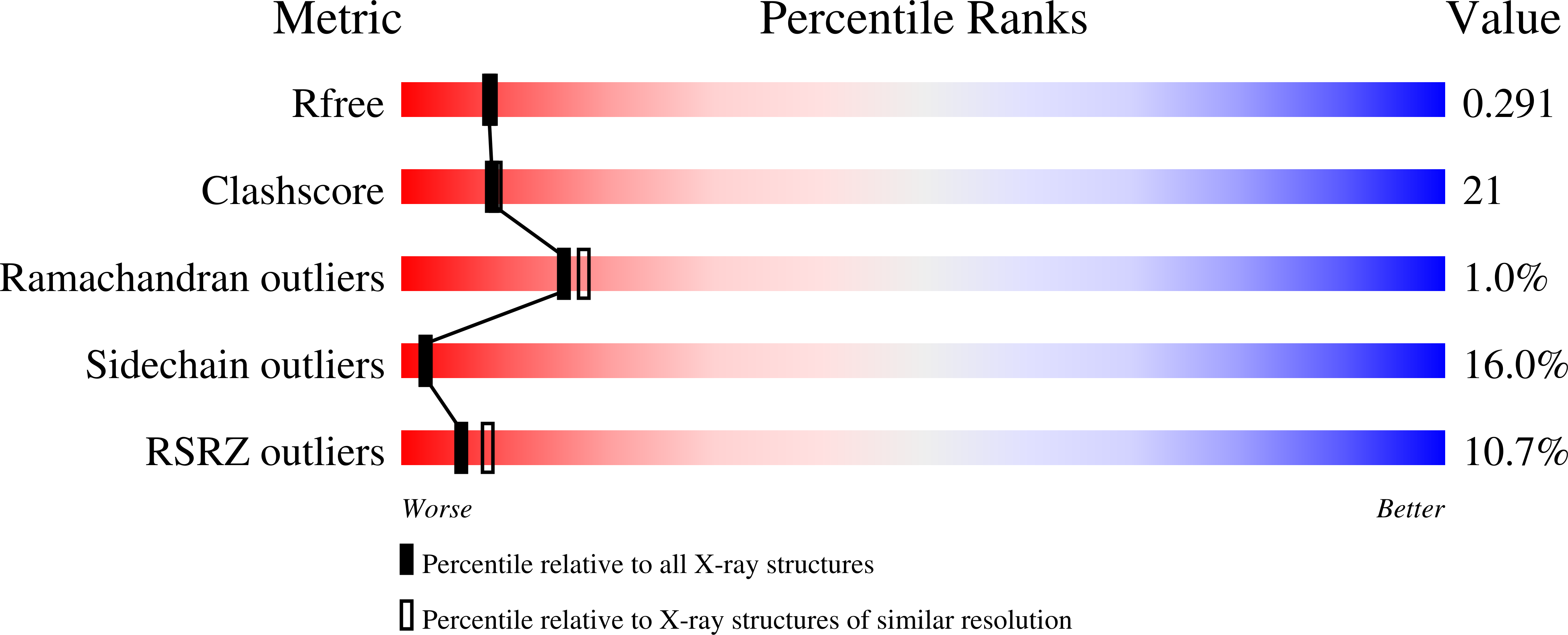
2FQM - PubMed Abstract:
In the replication cycle of nonsegmented negative-strand RNA viruses, the viral RNA-dependent RNA polymerase (L) recognizes a nucleoprotein (N)-enwrapped RNA template during the RNA polymerase reaction. The viral phosphoprotein (P) is a polymerase cofactor essential for this recognition. We report here the 2.3-angstroms-resolution crystal structure of the central domain (residues 107 to 177) of P from vesicular stomatitis virus. The fold of this domain consists of a beta hairpin, an alpha helix, and another beta hairpin. The alpha helix provides the stabilizing force for forming a homodimer, while the two beta hairpins add additional stabilization by forming a four-stranded beta sheet through domain swapping between two molecules. This central dimer positions the N- and C-terminal domains of P to interact with the N and L proteins, allowing the L protein to specifically recognize the nucleocapsid-RNA template and to progress along the template while concomitantly assembling N with nascent RNA. The interdimer interactions observed in the noncrystallographic packing may offer insight into the mechanism of the RNA polymerase processive reaction along the viral nucleocapsid-RNA template.
Organizational Affiliation:
Department of Microbiology, University of Alabama at Birmingham, 1025 18th Street South, Birmingham, Alabama 35294, USA.