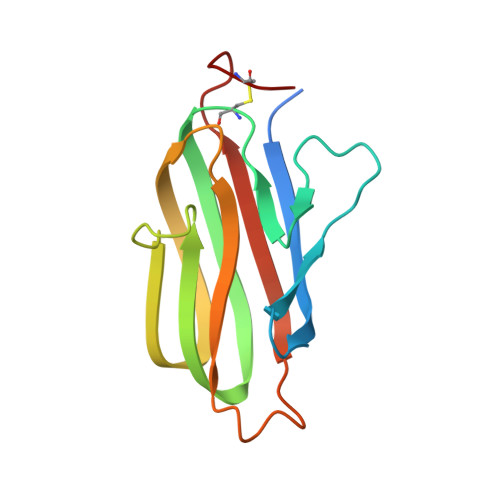
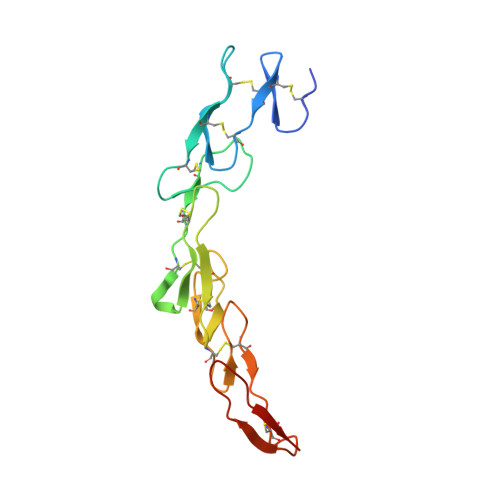
The Crystal Structure of the Costimulatory OX40-OX40L Complex.
Compaan, D.M., Hymowitz, S.G.(2006) Structure 14: 1321-1330
- PubMed: 16905106
- DOI: https://doi.org/10.1016/j.str.2006.06.015
- Primary Citation of Related Structures:
2HEV, 2HEW, 2HEY - PubMed Abstract:
OX40 is a T cell costimulator activated by OX40L. Blockade of the OX40L-OX40 interaction has ameliorative effects in animal models of T cell pathologies. In order to better understand the interaction between OX40 and OX40L, we have determined the crystal structure of murine OX40L and of the human OX40-OX40L complex at 1.45 and 2.4 A, respectively. These structures show that OX40L is an unusually small member of the tumor necrosis factor superfamily (TNFSF). The arrangement of the OX40L protomers forming the functional trimer is atypical and differs from that of other members by a 15 degrees rotation of each protomer with respect to the trimer axis, resulting in an open assembly. Site-directed changes of the interfacial residues of OX40L suggest this interface lacks a single "hot spot" and that instead, binding energy is dispersed over at least two distinct areas. These structures demonstrate the structural plasticity of TNFSF members and their interactions with receptors.
- Department of Protein Engineering, Genentech, Incorporated, 1 DNA Way, South San Francisco, California 94080, USA.
Organizational Affiliation: