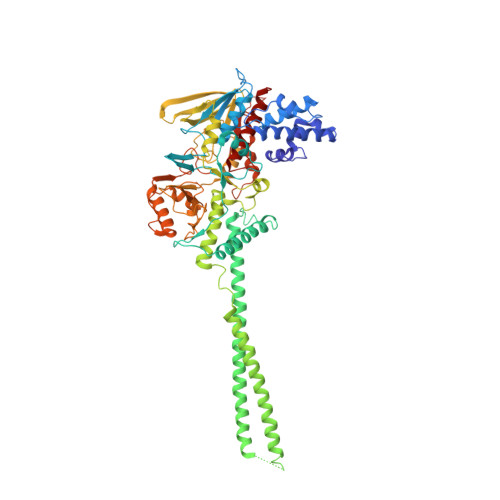
Crystal structure of human histone lysine-specific demethylase 1 (LSD1).
Chen, Y., Yang, Y., Wang, F., Wan, K., Yamane, K., Zhang, Y., Lei, M.(2006) Proc Natl Acad Sci U S A 103: 13956-13961
- PubMed: 16956976
- DOI: https://doi.org/10.1073/pnas.0606381103
- Primary Citation of Related Structures:
2HKO - PubMed Abstract:
Lysine-specific demethylase 1 (LSD1) was recently identified as the first histone demethylase that specifically demethylates monomethylated and dimethylated histone H3 at K4. It is a component of the CoREST and other corepressor complexes and plays an important role in silencing neuronal-specific genes in nonneuronal cells, but the molecular mechanisms of its action remain unclear. The 2.8-A-resolution crystal structure of the human LSD1 reveals that LSD1 defines a new subfamily of FAD-dependent oxidases. The active center of LSD1 is characterized by a remarkable 1,245-A3 substrate-binding cavity with a highly negative electrostatic potential. Although the protein core of LSD1 resembles other flavoenzymes, its enzymatic activity and functions require two additional structural modules: an N-terminal SWIRM domain important for protein stability and a large insertion in the catalytic domain indispensable both for the demethylase activity and the interaction with CoREST. These results provide a framework for further probing the catalytic mechanism and the functional roles of LSD1.
- Department of Biological Chemistry, University of Michigan Medical School, 5413 Medical Science I, 1301 Catherine Road, Ann Arbor, MI 48109-0606, USA.
Organizational Affiliation: