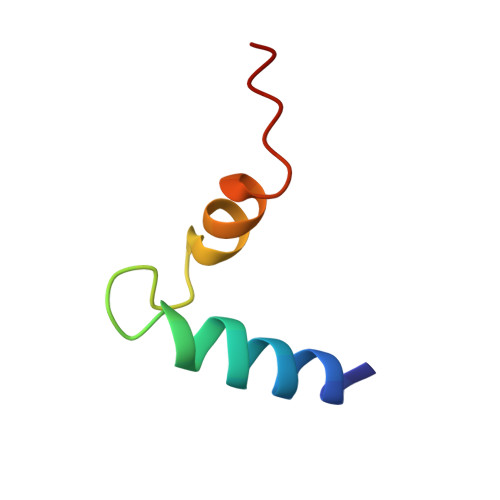
NMR solution structure of human cannabinoid receptor-1 helix 7/8 peptide: candidate electrostatic interactions and microdomain formation.
Tyukhtenko, S., Tiburu, E.K., Deshmukh, L., Vinogradova, O., Janero, D.R., Makriyannis, A.(2009) Biochem Biophys Res Commun 390: 441-446
- PubMed: 19766594
- DOI: https://doi.org/10.1016/j.bbrc.2009.09.053
- Primary Citation of Related Structures:
2KOE - PubMed Abstract:
We report the NMR solution structure of a synthetic 40-mer (T(377)-E(416)) that encompasses human cannabinoid receptor-1 (hCB1) transmembrane helix 7 (TMH7) and helix 8 (H8) [hCB1(TMH7/H8)] in 30% trifluoroethanol/H(2)O. Structural features include, from the peptide's amino terminus, a hydrophobic alpha-helix (TMH7); a loop-like, 11 residue segment featuring a pronounced Pro-kink within the conserved NPxxY motif; a short amphipathic alpha-helix (H8) orthogonal to TMH7 with cationic and hydrophobic amino-acid clusters; and an unstructured C-terminal end. The hCB1(TMH7/H8) NMR solution structure suggests multiple electrostatic amino-acid interactions, including an intrahelical H8 salt bridge and a hydrogen-bond network involving the peptide's loop-like region. Potential cation-pi and cation-phenolic OH interactions between Y(397) in the TMH7 NPxxY motif and R(405) in H8 are identified as candidate structural forces promoting interhelical microdomain formation. This microdomain may function as a flexible molecular hinge during ligand-induced hCB1 conformer transitions.
- Department of Chemistry and Chemical Biology, Northeastern University, Center for Drug Discovery, Boston, MA 02115-5000, USA.
Organizational Affiliation: