NMR evidence of GM1-induced conformational change of Substance P using isotropic bicelles
Gayen, A., Goswami, S.K., Mukhopadhyay, C.(2010) Biochim Biophys Acta
- PubMed: 20937248
- DOI: https://doi.org/10.1016/j.bbamem.2010.09.023
- Primary Citation of Related Structures:
2KS9, 2KSA, 2KSB - PubMed Abstract:
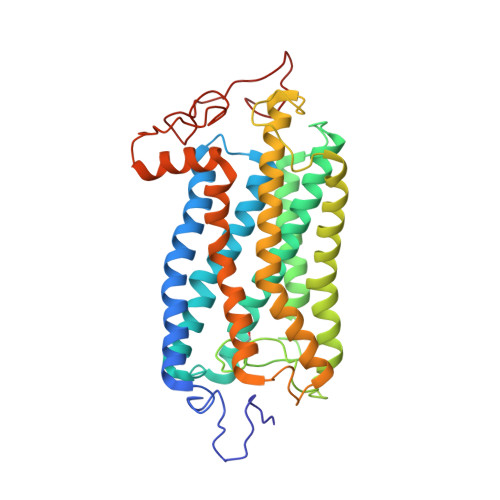
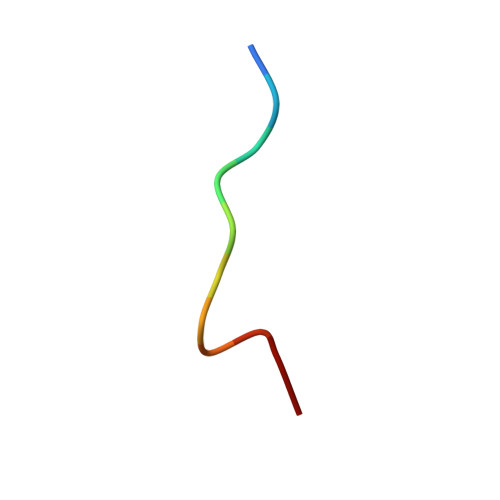
Substance P (SP) is one of the target neurotransmitters associated with diseases related to chronic inflammation, pain and depression. The selective receptor for SP, NK(1)R is located in the heterogeneous microdomains or caveolae in membrane. Gangliosides, specifically GM1, are markers of these heterogeneous sites. Also, gangliosides are considered as important regulatory elements in cell-cell recognition and cell signaling. In the present work, we describe the conformations of Substance P in the presence of ternary membrane systems containing GM1 at the physiological concentration. SP is mostly unstructured in water, but appears as extended 3(10) helical or turn III in isotropic bicelles, more pronounced in the presence of GM1. NMR results suggest that, in the GM1 containing bicelles, the peptide is more inserted into the membrane with its C-terminus, while N-terminus lies close to the membrane-water interface. The NMR-derived conformation of SP in GM1 bicelles is docked on homology modeled NK(1)R and resulting interactions satisfy reported mutagenesis, fluorescence, photo-affinity labeling and modeling data. The results highlight efficacy of GM1 in membrane in providing structure in an otherwise flexible neurotransmitter Substance P; thus providing indication that it may be useful also for other neurotransmitter peptides/proteins associated with membrane.
- Department of Chemistry, University of Calcutta, 92 APC Road, Kolkata 700009, India.
Organizational Affiliation: