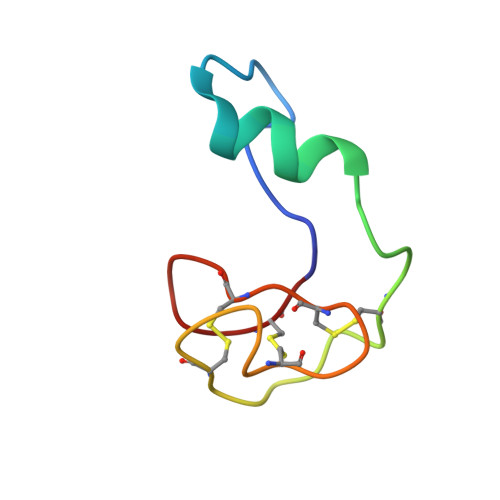
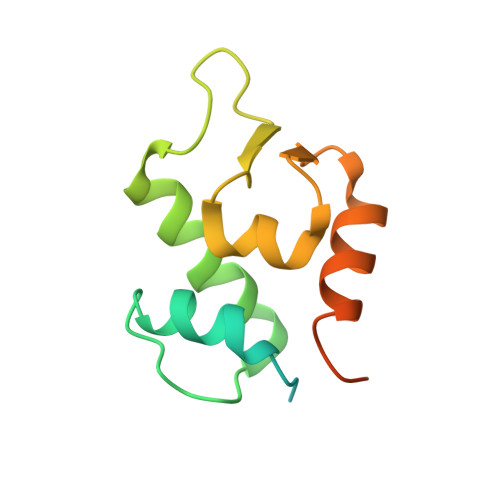
In Vivo Activation of the p53 Tumor Suppressor Pathway by an Engineered Cyclotide.
Ji, Y., Majumder, S., Millard, M., Borra, R., Bi, T., Elnagar, A.Y., Neamati, N., Shekhtman, A., Camarero, J.A.(2013) J Am Chem Soc 135: 11623-11633
- PubMed: 23848581
- DOI: https://doi.org/10.1021/ja405108p
- Primary Citation of Related Structures:
2M86 - PubMed Abstract:
The overexpression of Hdm2 and HdmX is a common mechanism used by many tumor cells to inactive the p53 tumor suppressor pathway promoting cell survival. Targeting Hdm2 and HdmX has emerged as a validated therapeutic strategy for treating cancers with wild-type p53. Small linear peptides mimicking the N-terminal fragment of p53 have been shown to be potent Hdm2/HdmX antagonists. The potential therapeutic use of these peptides, however, is limited by their poor stability and bioavailability. Here, we report the engineering of the cyclotide MCoTI-I to efficiently antagonize intracellular p53 degradation. The resulting cyclotide MCo-PMI was able to bind with low nanomolar affinity to both Hdm2 and HdmX, showed high stability in human serum, and was cytotoxic to wild-type p53 cancer cell lines by activating the p53 tumor suppressor pathway both in vitro and in vivo. These features make the cyclotide MCoTI-I an optimal scaffold for targeting intracellular protein-protein interactions.
- Department of Pharmacology and Pharmaceutical Sciences, University of Southern California, Los Angeles, CA 90033, USA.
Organizational Affiliation: