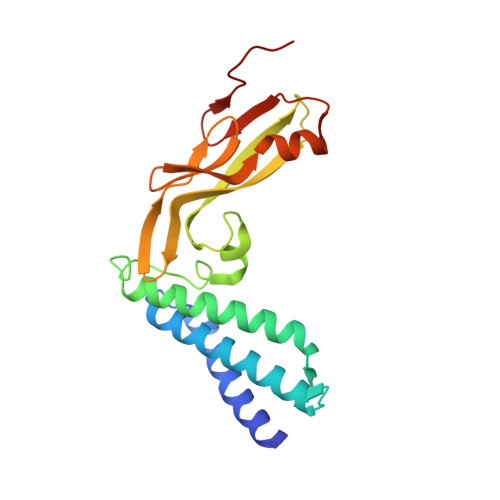
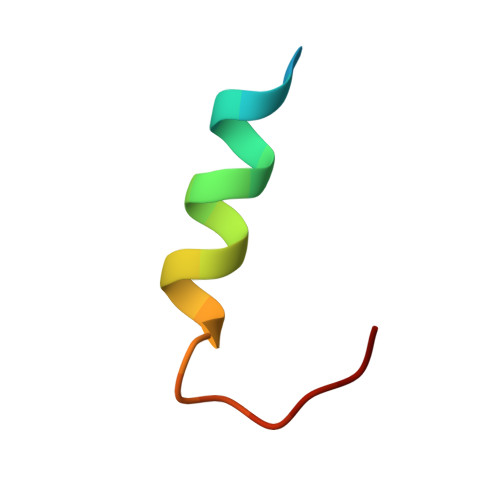
Structure of the Papillomavirus DNA-Tethering Complex E2:Brd4 and a Peptide that Ablates HPV Chromosomal Association.
Abbate, E.A., Voitenleitner, C., Botchan, M.R.(2006) Mol Cell 24: 877-889
- PubMed: 17189190
- DOI: https://doi.org/10.1016/j.molcel.2006.11.002
- Primary Citation of Related Structures:
2NNU - PubMed Abstract:
Many DNA viruses that are latent in dividing cells are noncovalent passengers on mitotic chromosomes and require specific viral-encoded and cellular factors for this activity. The chromosomal protein Brd4 is implicated in the hitchhiking of bovine papillomavirus-1 (BPV-1), and the viral protein E2 binds to both plasmids and Brd4. Here, we present the X-ray crystal structure of the carboxy-terminal domain of Brd4 in complex with HPV-16 E2, and with this information have developed a Brd4-Tat fusion protein that is efficiently taken up by different transformed cells harboring HPV plasmids. In cells treated with these fusion proteins for only 2 hr and arrested in metaphase, the HPV DNA, either HPV-16 or -31, is displaced from mitotic chromosomes. Mutant Brd4 peptides are deficient in ablating this association. We suggest that such peptides may lead to the development of inhibitors of latency for many, if not all, papillomaviruses.
- Department of Molecular and Cell Biology, Division of Biochemistry and Molecular Biology, University of California, Berkeley, Berkeley, California 94720, USA.
Organizational Affiliation: