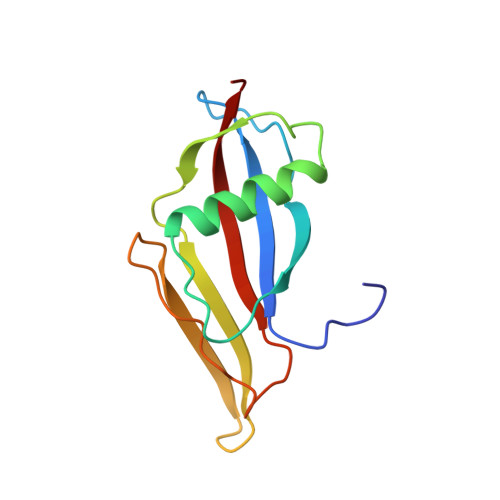
Three-dimensional structure of staphylokinase, a plasminogen activator with therapeutic potential.
Rabijns, A., De Bondt, H.L., De Ranter, C.(1997) Nat Struct Biol 4: 357-360
- PubMed: 9145104
- DOI: https://doi.org/10.1038/nsb0597-357
- Primary Citation of Related Structures:
2SAK - PubMed Abstract:
The three-dimensional structure of staphylokinase has been determined at 1.8 A. The puntative site of interaction with plasminogen was identified and epitopes were mapped.