Identification and biological evaluation of a series of 1H-benzo[de]isoquinoline-1,3(2H)-diones as hepatitis C virus NS5B polymerase inhibitors.
Ontoria, J.M., Rydberg, E.H., Di Marco, S., Tomei, L., Attenni, B., Malancona, S., Martin Hernando, J.I., Gennari, N., Koch, U., Narjes, F., Rowley, M., Summa, V., Carroll, S.S., Olsen, D.B., De Francesco, R., Altamura, S., Migliaccio, G., Carfi, A.(2009) J Med Chem 52: 5217-5227
- PubMed: 19877603
- DOI: https://doi.org/10.1021/jm900517t
- Primary Citation of Related Structures:
2WHO - PubMed Abstract:
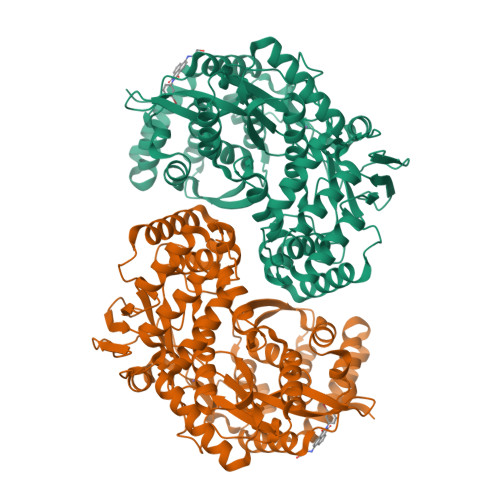
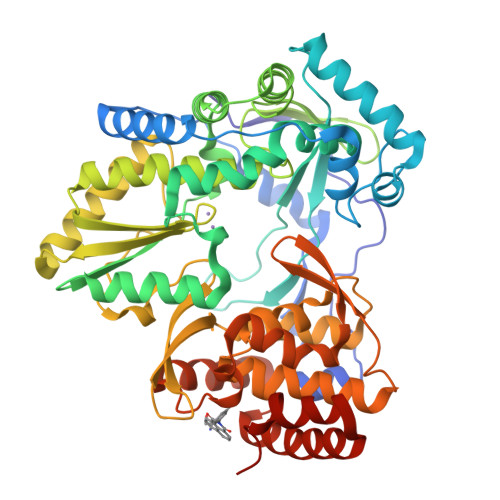
The hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase (RdRp) plays a central role in virus replication. NS5B has no functional equivalent in mammalian cells and, as a consequence, is an attractive target for inhibition. Herein, we present 1H-benzo[de]isoquinoline-1,3(2H)-diones as a new series of selective inhibitors of HCV NS5B polymerase. The HTS hit 1 shows submicromolar potency in two different HCV replicons (1b and 2b) and displays no activity on other polymerases (HIV-RT, Polio-pol, GBV-b-pol). These inhibitors act during the pre-elongation phase by binding to NS5B non-nucleoside binding site Thumb Site II as demonstrated by crystal structure of compound 1 with the DeltaC55-1b and DeltaC21-2b enzymes and by mutagenesis studies. SAR in this new series reveals inhibitors, such as 20, with low micromolar activity in the HCV replicon and with good activity/toxicity window in cells.
Organizational Affiliation:
Istituto Di Ricerche Di Biologia Molecolare, P. Angeletti, S.p.A. (IRBM-MRL Rome), Via Pontina Km 30,600, I-00040 Pomezia, Italy. jesus_ontoria@merck.com