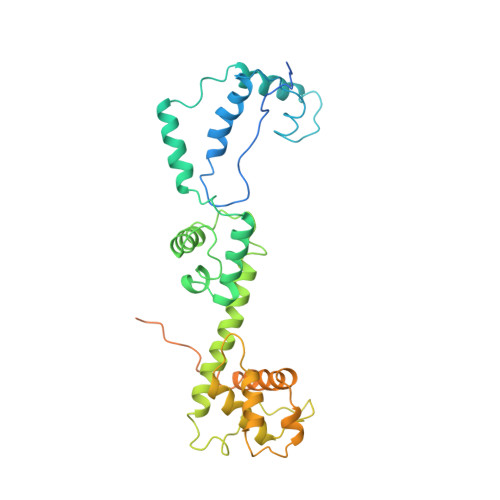
Structure and Mechanism of the Chromatin Remodelling Factor Isw1A.
Yamada, K., Frouws, T.D., Angst, B., Fitzgerald, D.J., Deluca, C., Schimmele, K., Sargent, D.F., Richmond, T.J.(2011) Nature 472: 448
- PubMed: 21525927
- DOI: https://doi.org/10.1038/nature09947
- Primary Citation of Related Structures:
2Y9Y, 2Y9Z - PubMed Abstract:
Site-specific recognition of DNA in eukaryotic organisms depends on the arrangement of nucleosomes in chromatin. In the yeast Saccharomyces cerevisiae, ISW1a and related chromatin remodelling factors are implicated in establishing the nucleosome repeat during replication and altering nucleosome position to affect gene activity. Here we have solved the crystal structures of S. cerevisiae ISW1a lacking its ATPase domain both alone and with DNA bound at resolutions of 3.25 Å and 3.60 Å, respectively, and we have visualized two different nucleosome-containing remodelling complexes using cryo-electron microscopy. The composite X-ray and electron microscopy structures combined with site-directed photocrosslinking analyses of these complexes suggest that ISW1a uses a dinucleosome substrate for chromatin remodelling. Results from a remodelling assay corroborate the dinucleosome model. We show how a chromatin remodelling factor could set the spacing between two adjacent nucleosomes acting as a 'protein ruler'.
Organizational Affiliation:
ETH Zürich, Institute of Molecular Biology and Biophysics, Schafmattstr. 20, CH-8093 Zürich, Switzerland.