Optimization of novel di-substituted cyclohexylbenzamide derivatives as potent 11 beta-HSD1 inhibitors.
McMinn, D.L., Rew, Y., Sudom, A., Caille, S., Degraffenreid, M., He, X., Hungate, R., Jiang, B., Jaen, J., Julian, L.D., Kaizerman, J., Novak, P., Sun, D., Tu, H., Ursu, S., Walker, N.P., Yan, X., Ye, Q., Wang, Z., Powers, J.P.(2009) Bioorg Med Chem Lett 19: 1446-1450
- PubMed: 19185488
- DOI: https://doi.org/10.1016/j.bmcl.2009.01.026
- Primary Citation of Related Structures:
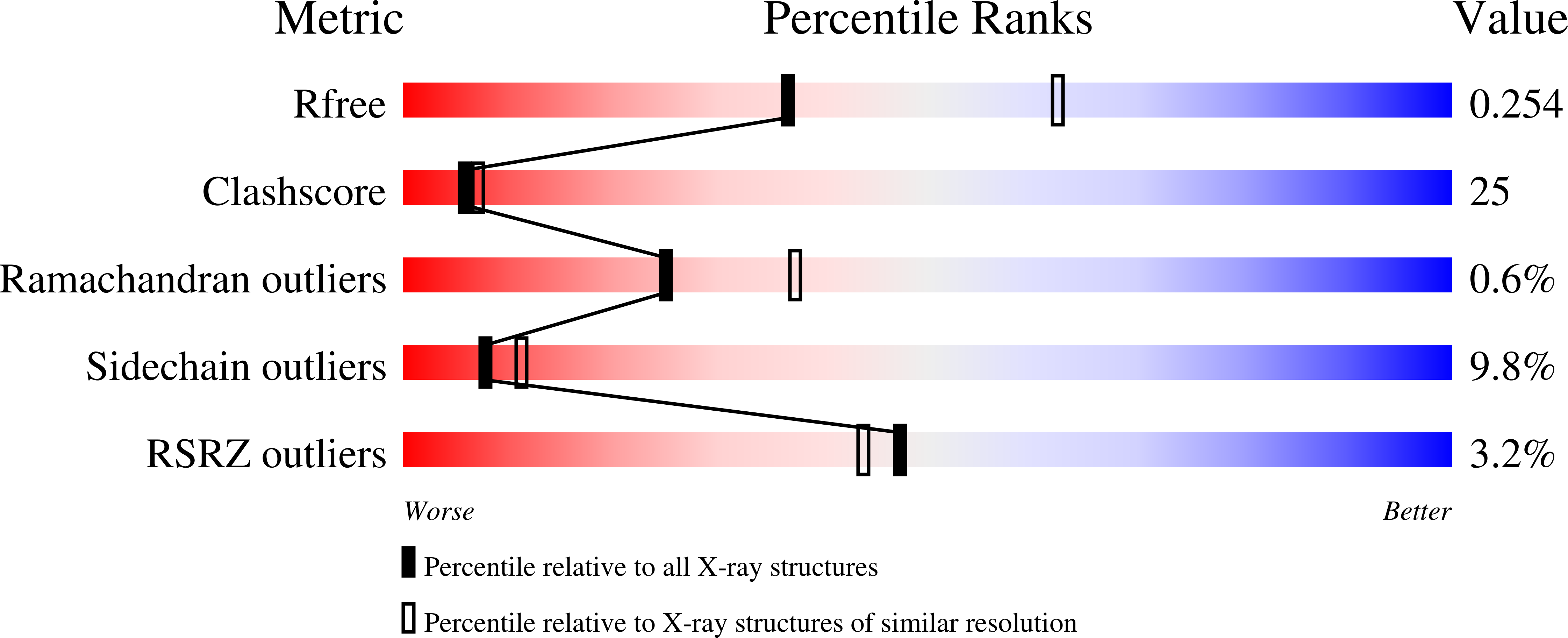
3FCO - PubMed Abstract:
Novel 4,4-disubstituted cyclohexylbenzamide inhibitors of 11beta-HSD1 were optimized to account for liabilities relating to in vitro pharmacokinetics, cytotoxicity and protein-related shifts in potency. A representative compound showing favorable in vivo pharmacokinetics was found to be an efficacious inhibitor of 11beta-HSD1 in a rat pharmacodynamic model (ED(50)=10mg/kg).
- Amgen, Inc., 1120 Veterans Boulevard, South San Francisco, CA 94080, USA.
Organizational Affiliation: