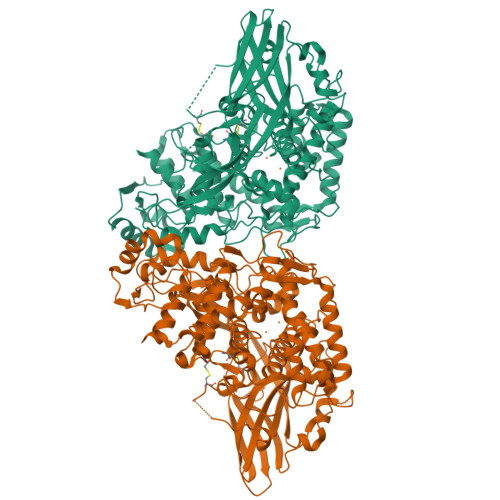
Crystal structure of Manduca sexta prophenoloxidase provides insights into the mechanism of type 3 copper enzymes.
Li, Y., Wang, Y., Jiang, H., Deng, J.(2009) Proc Natl Acad Sci U S A 106: 17002-17006
- PubMed: 19805072
- DOI: https://doi.org/10.1073/pnas.0906095106
- Primary Citation of Related Structures:
3HHS - PubMed Abstract:
Arthropod phenoloxidase (PO) generates quinones and other toxic compounds to sequester and kill pathogens during innate immune responses. It is also involved in wound healing and other physiological processes. Insect PO is activated from its inactive precursor, prophenoloxidase (PPO), by specific proteolysis via a serine protease cascade. Here, we report the crystal structure of PPO from a lepidopteran insect at a resolution of 1.97 A, which is the initial structure for a PPO from the type 3 copper protein family. Manduca sexta PPO is a heterodimer consisting of 2 homologous polypeptide chains, PPO1 and PPO2. The active site of each subunit contains a canonical type 3 di-nuclear copper center, with each copper ion coordinated with 3 structurally conserved histidines. The acidic residue Glu-395 located at the active site of PPO2 may serve as a general base for deprotonation of monophenolic substrates, which is key to the ortho-hydroxylase activity of PO. The structure provides unique insights into the mechanism by which type 3 copper proteins differ in their enzymatic activities, albeit sharing a common active center. A drastic change in electrostatic surface induced on cleavage at Arg-51 allows us to propose a model for localized PPO activation in insects.
Organizational Affiliation:
Department of Biochemistry, Oklahoma State University, Stillwater, OK 74078, USA.