The X-ray Crystal Structure of PCSK9 in Complex with the LDL receptor
Li, J., Gavigan, J.A., Zheng, G., Huang, W., Yowe, D., Geisse, S., Harris, J.L., Lesley, S.A., Spraggon, G.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Proprotein convertase subtilisin/kexin type 9 | 124 | Homo sapiens | Mutation(s): 0 Gene Names: NARC1, PCSK9, PSEC0052 EC: 3.4.21 | 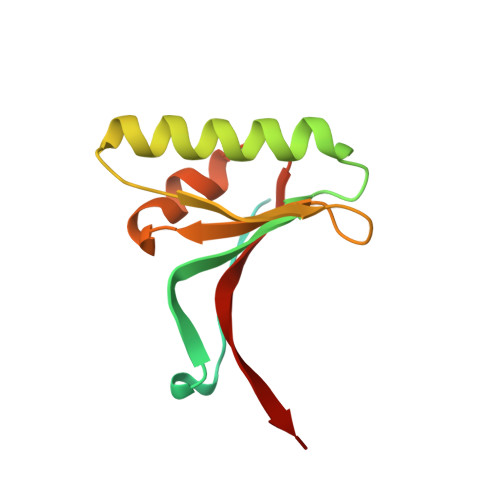 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8NBP7 (Homo sapiens) Explore Q8NBP7 Go to UniProtKB: Q8NBP7 | |||||
PHAROS: Q8NBP7 GTEx: ENSG00000169174 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8NBP7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Proprotein convertase subtilisin/kexin type 9 | 546 | Homo sapiens | Mutation(s): 1 Gene Names: NARC1, PCSK9, PSEC0052 EC: 3.4.21 |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8NBP7 (Homo sapiens) Explore Q8NBP7 Go to UniProtKB: Q8NBP7 | |||||
PHAROS: Q8NBP7 GTEx: ENSG00000169174 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8NBP7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Low-density lipoprotein receptor | 791 | Homo sapiens | Mutation(s): 0 Gene Names: LDLR | 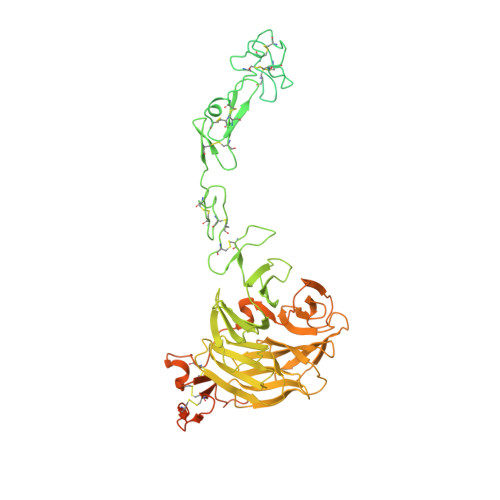 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01130 (Homo sapiens) Explore P01130 Go to UniProtKB: P01130 | |||||
PHAROS: P01130 GTEx: ENSG00000130164 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01130 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CA Query on CA | D [auth C], E [auth C], F [auth C] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 322.906 | α = 90 |
| b = 322.906 | β = 90 |
| c = 76.733 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHASER | phasing |
| PHENIX | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |