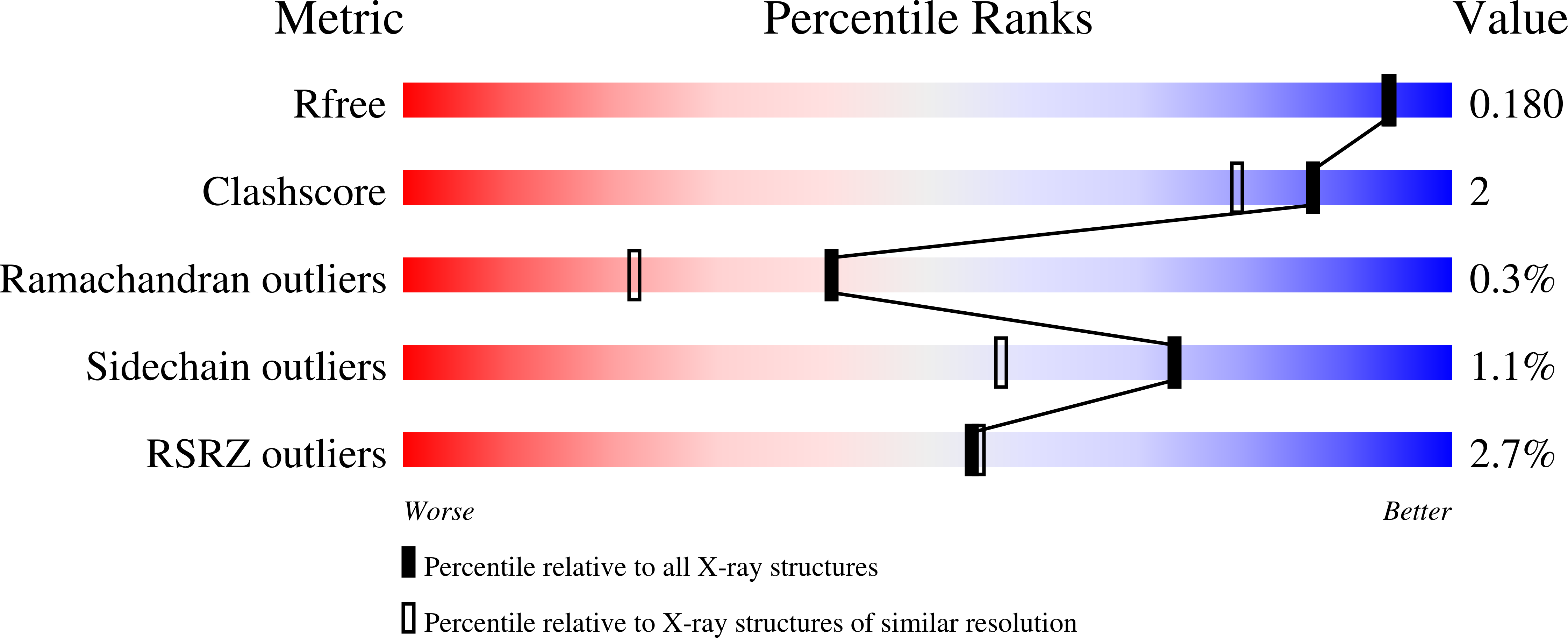
Pressure-response analysis of anesthetic gases xenon and nitrous oxide on urate oxidase: a crystallographic study.
Marassio, G., Prange, T., David, H.N., Sopkova-de Oliveira Santos, J., Gabison, L., Delcroix, N., Abraini, J.H., Colloc'h, N.(2011) FASEB J 25: 2266-2275
- PubMed: 21421845
- DOI: https://doi.org/10.1096/fj.11-183046
- Primary Citation of Related Structures:
3PJK, 3PK3, 3PK4, 3PK5, 3PK6, 3PK8, 3PKF, 3PKG, 3PKH, 3PKK, 3PKL, 3PKS, 3PKT, 3PKU, 3PLE, 3PLG, 3PLH, 3PLI, 3PLJ, 3PLM - PubMed Abstract:
The remarkably safe anesthetics xenon (Xe) and, to lesser extent, nitrous oxide (N(2)O) possess neuroprotective properties in preclinical studies. To investigate the mechanisms of pharmacological action of these gases, which are still poorly known, we performed both crystallography under a large range of gas pressure and biochemical studies on urate oxidase, a prototype of globular gas-binding proteins whose activity is modulated by inert gases. We show that Xe and N(2)O bind to, compete for, and expand the volume of a hydrophobic cavity located just behind the active site of urate oxidase and further inhibit urate oxidase enzymatic activity. By demonstrating a significant relationship between the binding and biochemical effects of Xe and N(2)O, given alone or in combination, these data from structure to function highlight the mechanisms by which chemically and metabolically inert gases can alter protein function and produce their pharmacological effects. Interestingly, the effects of a Xe:N(2)O equimolar mixture were found to be equivalent to those of Xe alone, thereby suggesting that gas mixtures containing Xe and N(2)O could be an alternative and efficient neuroprotective strategy to Xe alone, whose widespread clinical use is limited due to the cost of production and availability of this gas.
Organizational Affiliation:
Equipe de Recherche Technologique Interne (ERTi) 1083, Centre National de la Recherche Scientifique (CNRS), Centre Cyceron, Caen, France.