The initiation of GTP hydrolysis by the G-domain of FeoB: insights from a transition-state complex structure
Ash, M.R., Maher, M.J., Guss, J.M., Jormakka, M.(2011) PLoS One 6: e23355-e23355
- PubMed: 21858085
- DOI: https://doi.org/10.1371/journal.pone.0023355
- Primary Citation of Related Structures:
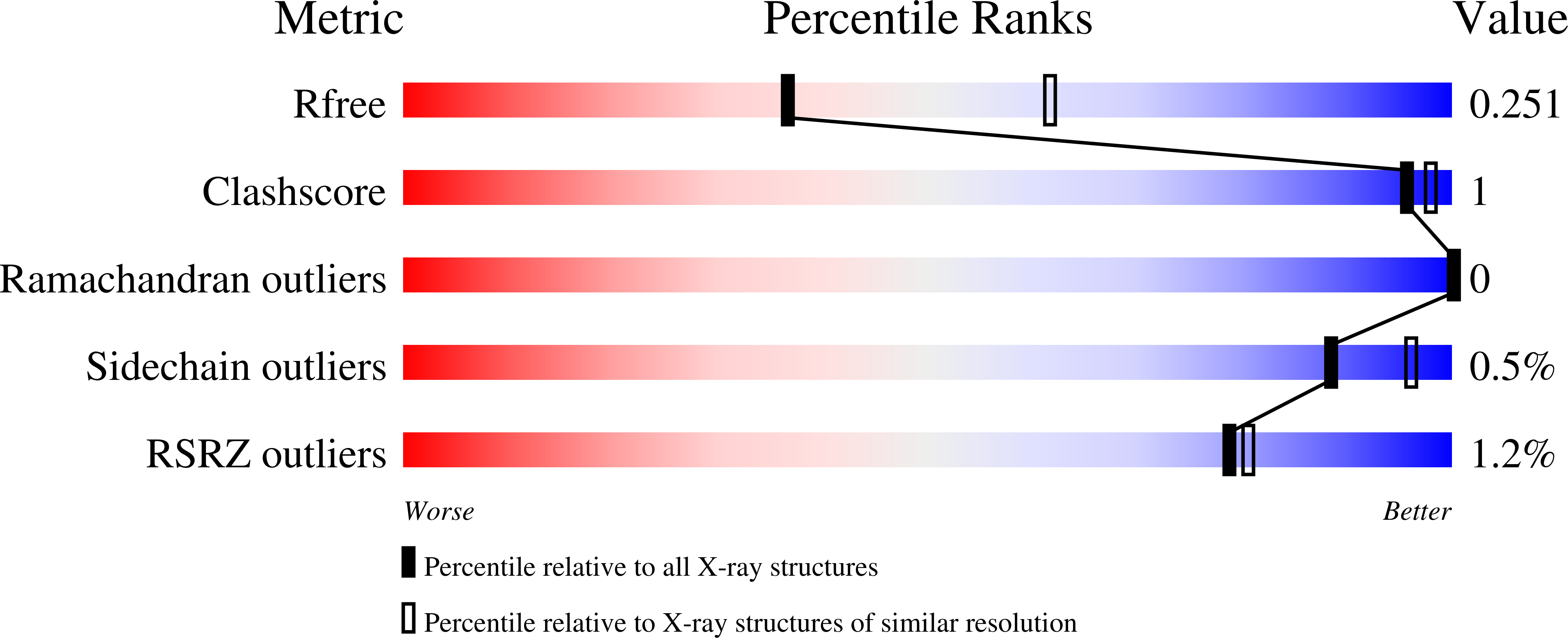
3SS8 - PubMed Abstract:
The polytopic membrane protein FeoB is a ferrous iron transporter in prokaryotes. The protein contains a potassium-activated GTPase domain that is essential in regulating the import of iron and conferring virulence to many disease-causing bacteria. However, the mechanism by which the G-domain of FeoB hydrolyzes GTP is not well understood. In particular, it is not yet known how the pivotal step in GTP hydrolysis is achieved: alignment of a catalytic water molecule. In the current study, the crystal structure of the soluble domains from Streptococcus thermophilus FeoB (NFeoB(St)) in complex with the activating potassium ion and a transition-state analogue, GDP⋅AlF(4) (-), reveals a novel mode of water alignment involving contacts with the protein backbone only. In parallel to the structural studies, a series of seven mutant proteins were constructed that targeted conserved residues at the active site of NFeoB(St), and the nucleotide binding and hydrolysis properties of these were measured and compared to the wild-type protein. The results show that mutations in Thr35 abolish GTPase activity of the protein, while other conserved residues (Tyr58, Ser64, Glu66 and Glu67) are not required for water alignment by NFeoB(St). Together with the crystal structure, the findings suggest a new mechanism for hydrolysis initiation in small G-proteins, in which the attacking water molecule is aligned by contacts with the protein backbone only.
Organizational Affiliation:
School of Molecular Bioscience, University of Sydney, New South Wales, Australia.