Dttp Inhibition of the Bifunctional Dctp Deaminase- Dutpase from Mycobacterium Tuberculosis is Ph Dependent: Kinetic Analyses and Crystal Structure of A115V Variant
Lovgreen, M.N., Harris, P., Ucar, E., Willemoes, M.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DEOXYCYTIDINE TRIPHOSPHATE DEAMINASE | 190 | Mycobacterium tuberculosis H37Rv | Mutation(s): 1 EC: 3.5.4.13 (PDB Primary Data), 3.5.4.30 (UniProt) | 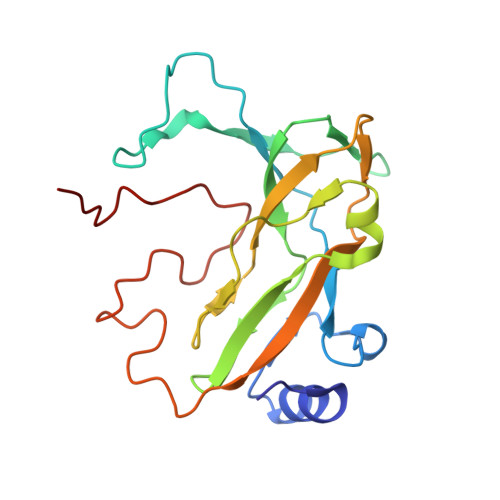 | |
UniProt | |||||
Find proteins for P9WP17 (Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv)) Explore P9WP17 Go to UniProtKB: P9WP17 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P9WP17 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TTP Query on TTP | BA [auth H] EA [auth I] GA [auth J] IA [auth K] KA [auth L] | THYMIDINE-5'-TRIPHOSPHATE C10 H17 N2 O14 P3 NHVNXKFIZYSCEB-XLPZGREQSA-N |  | ||
| MG Query on MG | AA [auth G] CA [auth H] DA [auth H] FA [auth I] HA [auth J] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 56.59 | α = 90 |
| b = 179.51 | β = 96.28 |
| c = 99.81 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| XSCALE | data scaling |