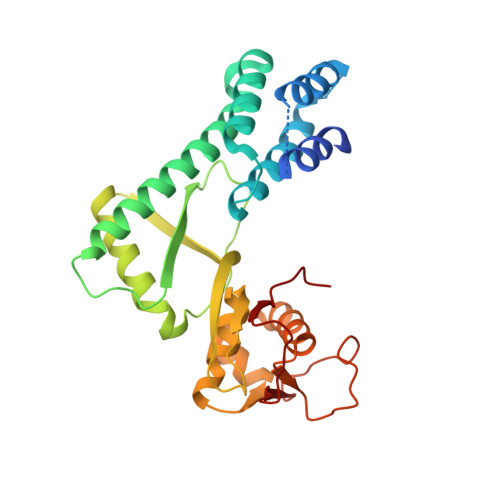
Structure of a Glomulin-RBX1-CUL1 Complex: Inhibition of a RING E3 Ligase through Masking of Its E2-Binding Surface.
Duda, D.M., Olszewski, J.L., Tron, A.E., Hammel, M., Lambert, L.J., Waddell, M.B., Mittag, T., Decaprio, J.A., Schulman, B.A.(2012) Mol Cell 47: 371-382
- PubMed: 22748924
- DOI: https://doi.org/10.1016/j.molcel.2012.05.044
- Primary Citation of Related Structures:
4F52 - PubMed Abstract:
The approximately 300 human cullin-RING ligases (CRLs) are multisubunit E3s in which a RING protein, either RBX1 or RBX2, recruits an E2 to catalyze ubiquitination. RBX1-containing CRLs also can bind Glomulin (GLMN), which binds RBX1's RING domain, regulates the RBX1-CUL1-containing SCF(FBW7) complex, and is disrupted in the disease Glomuvenous Malformation. Here we report the crystal structure of a complex between GLMN, RBX1, and a fragment of CUL1. Structural and biochemical analyses reveal that GLMN adopts a HEAT-like repeat fold that tightly binds the E2-interacting surface of RBX1, inhibiting CRL-mediated chain formation by the E2 CDC34. The structure explains the basis for GLMN's selectivity toward RBX1 over RBX2, and how disease-associated mutations disrupt GLMN-RBX1 interactions. Our study reveals a mechanism for RING E3 ligase regulation, whereby an inhibitor blocks E2 access, and raises the possibility that other E3s are likewise controlled by cellular proteins that mask E2-binding surfaces to mediate inhibition.
- Department of Structural Biology, St. Jude Children's Research Hospital, Memphis TN 38105, USA.
Organizational Affiliation: