Crystal Structure of the Urokinase
Kang, Y.N., Stuckey, J.A., Nienaber, V., Giranda, V.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Urokinase-type plasminogen activator | 246 | Homo sapiens | Mutation(s): 2 Gene Names: PLAU EC: 3.4.21.73 | 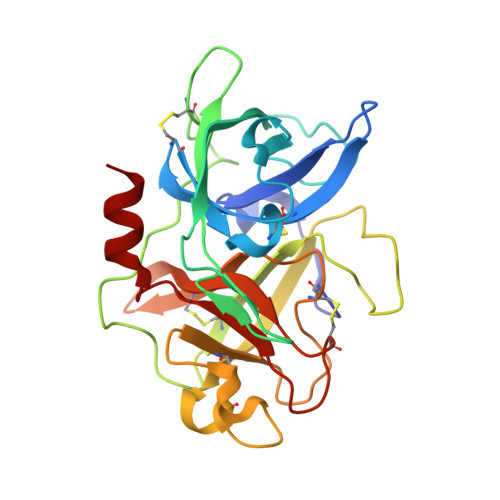 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00749 (Homo sapiens) Explore P00749 Go to UniProtKB: P00749 | |||||
PHAROS: P00749 GTEx: ENSG00000122861 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00749 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 6 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 15P Query on 15P | J [auth A] | POLYETHYLENE GLYCOL (N=34) C69 H140 O35 VUYXVWGKCKTUMF-UHFFFAOYSA-N |  | ||
| 4UP Query on 4UP | B [auth A] | 6-[(2S,3S)-3-phenyloxiran-2-yl]naphthalene-2-carboximidamide C19 H16 N2 O RZYGGGSOUBZFKW-ROUUACIJSA-N |  | ||
| SIN Query on SIN | D [auth A] | SUCCINIC ACID C4 H6 O4 KDYFGRWQOYBRFD-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | C [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | E [auth A] F [auth A] G [auth A] H [auth A] I [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| ACT Query on ACT | L [auth A], M [auth A], N [auth A], O [auth A], P [auth A] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 55.1 | α = 90 |
| b = 52.4 | β = 90 |
| c = 80.25 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| BUSTER-TNT | refinement |
| PDB_EXTRACT | data extraction |
| BUSTER | refinement |