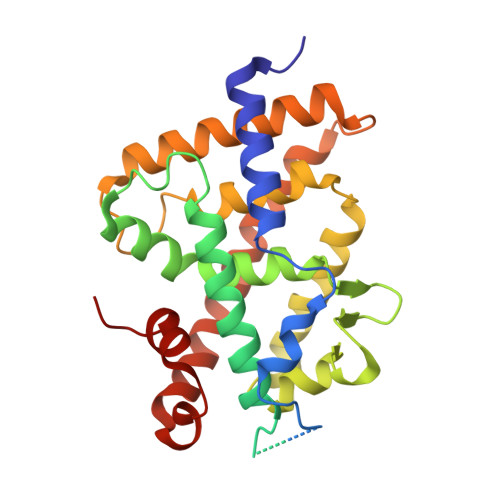
Diastereotopic and deuterium effects in gemini.
Maehr, H., Rochel, N., Lee, H.J., Suh, N., Uskokovic, M.R.(2013) J Med Chem 56: 3878-3888
- PubMed: 23566225
- DOI: https://doi.org/10.1021/jm400032t
- Primary Citation of Related Structures:
4IA1, 4IA2, 4IA3, 4IA7 - PubMed Abstract:
Changing the geminal methyl groups on 1α,25-dihydroxyvitamin D3 and its analogues to the deuterio versions generally improves the bioactivity. Derivatives of 1α,25-dihydroxyvitamin D3 with two chains emanating at C20, commonly referred to as gemini, are subject to the same phenomenon. Additionally, gemini with different side chains are susceptible to bioactivity differentials where the C17-C20 threo configuration usually imparts higher activity than the corresponding erythro arrangement. In an effort to analyze the deuterium effect on gemini with minimal diastereotopic distortion, we synthesized gemini with equal side chains but introduced deuterium diastereospecifically on either chain. We solved the crystal structures of these compounds in the zebra fish zVDR ligand binding domain as complexes with NCoA-2 coactivator peptide and correlated the findings with growth inhibition in a breast cancer cell line.
- Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, 164 Frelinghuysen Road, Piscataway, New Jersey 08854, USA. hubert.maehr@verizon.net
Organizational Affiliation: