Crystal structure of an Anticalin with specific blocking activity towards human vascular endothelial growth factor (VEGF) reveals plasticity of the lipocalin fold
Giese, T., Skerra, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lipocalin-1 | A, D [auth B] | 152 | Homo sapiens | Mutation(s): 27 Gene Names: engineered variant, LCN1 | 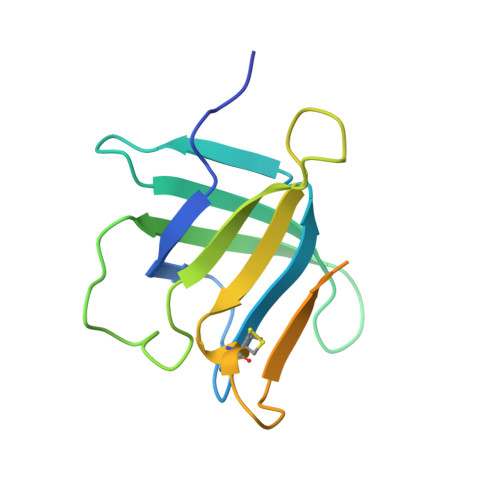 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P31025 (Homo sapiens) Explore P31025 Go to UniProtKB: P31025 | |||||
PHAROS: P31025 GTEx: ENSG00000160349 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P31025 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vascular endothelial growth factor A | B [auth C], C [auth D] | 112 | Homo sapiens | Mutation(s): 0 Gene Names: VEGFA, VEGF | 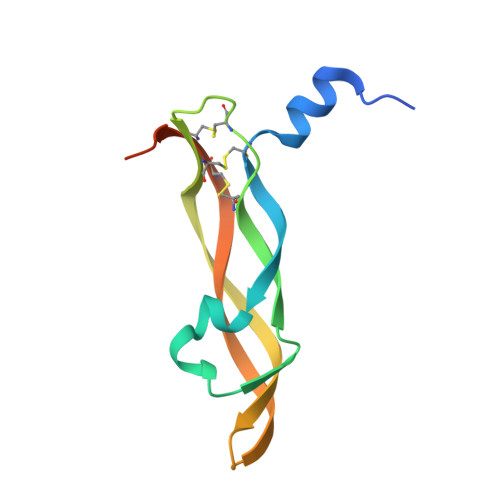 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P15692 (Homo sapiens) Explore P15692 Go to UniProtKB: P15692 | |||||
PHAROS: P15692 GTEx: ENSG00000112715 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P15692 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| OMA Query on OMA | E [auth A], J [auth B] | 10-{(1R,2R)-2-[(2E)-hex-2-en-1-yl]cyclopropyl}decanoic acid C19 H34 O2 MGOIQHKBDDJUJK-XVSUHRFUSA-N |  | ||
| SO4 Query on SO4 | F [auth C], G [auth C], H [auth D] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| ACT Query on ACT | I [auth D] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 88.21 | α = 90 |
| b = 88.21 | β = 90 |
| c = 103.421 | γ = 120 |
| Software Name | Purpose |
|---|---|
| XSCALE | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MAR345dtb | data collection |
| XDS | data reduction |
| Auto-Rickshaw | phasing |