A structural mechanism for bacterial autotransporter glycosylation by a dodecameric heptosyltransferase family
Yao, Q., Lu, Q., Wan, X., Song, F., Xu, Y., Hu, M., Zamyatina, A., Liu, X., Huang, N., Zhu, P., Shao, F.(2014) Elife 3
- PubMed: 25310236
- DOI: https://doi.org/10.7554/eLife.03714
- Primary Citation of Related Structures:
4RAP, 4RB4 - PubMed Abstract:
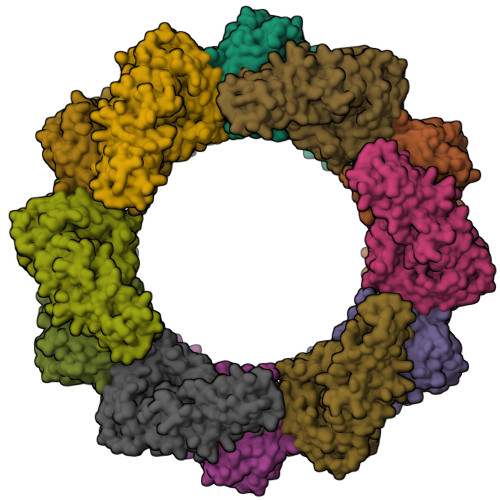
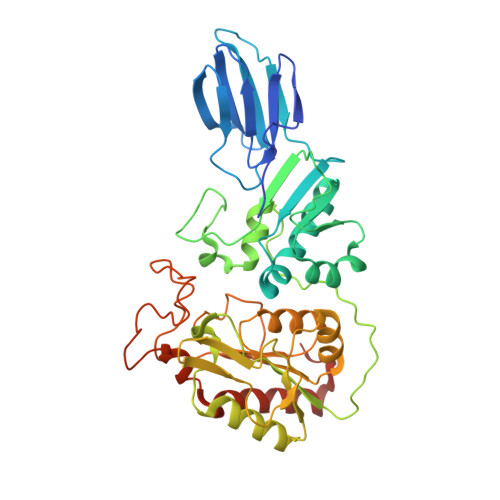
A large group of bacterial virulence autotransporters including AIDA-I from diffusely adhering E. coli (DAEC) and TibA from enterotoxigenic E. coli (ETEC) require hyperglycosylation for functioning. Here we demonstrate that TibC from ETEC harbors a heptosyltransferase activity on TibA and AIDA-I, defining a large family of bacterial autotransporter heptosyltransferases (BAHTs). The crystal structure of TibC reveals a characteristic ring-shape dodecamer. The protomer features an N-terminal β-barrel, a catalytic domain, a β-hairpin thumb, and a unique iron-finger motif. The iron-finger motif contributes to back-to-back dimerization; six dimers form the ring through β-hairpin thumb-mediated hand-in-hand contact. The structure of ADP-D-glycero-β-D-manno-heptose (ADP-D,D-heptose)-bound TibC reveals a sugar transfer mechanism and also the ligand stereoselectivity determinant. Electron-cryomicroscopy analyses uncover a TibC-TibA dodecamer/hexamer assembly with two enzyme molecules binding to one TibA substrate. The complex structure also highlights a high efficient hyperglycosylation of six autotransporter substrates simultaneously by the dodecamer enzyme complex.
Organizational Affiliation:
Dr Feng Shao's Laboratory, National Institute of Biological Sciences, Beijing, China.