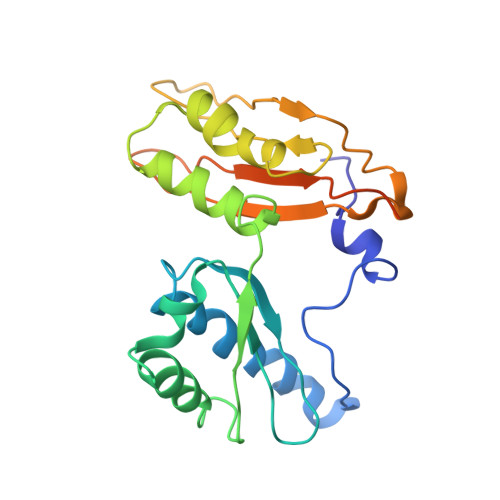
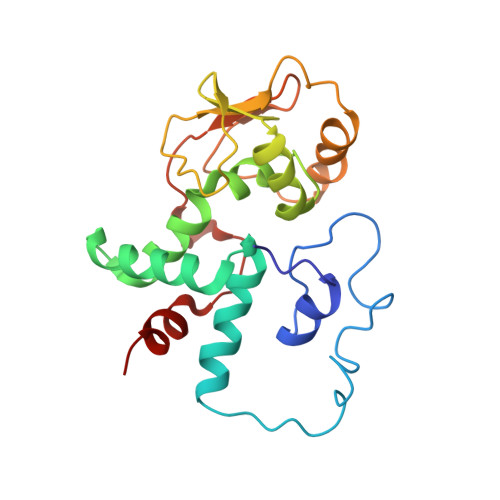
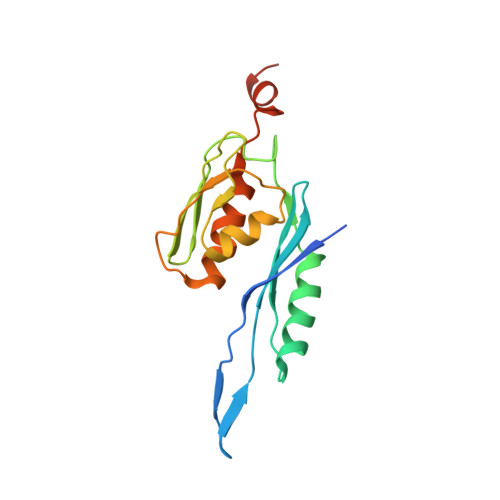
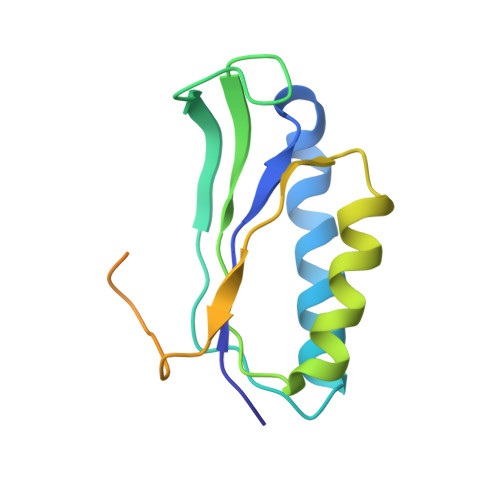
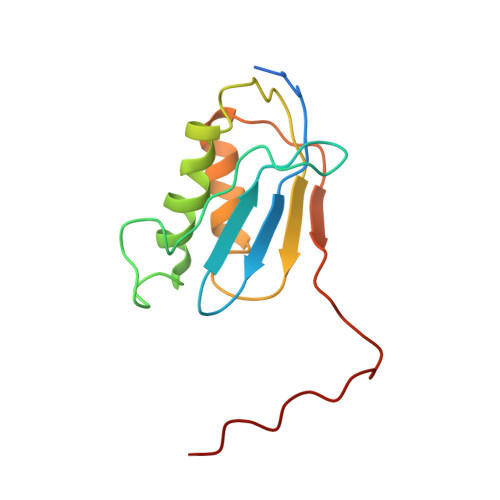
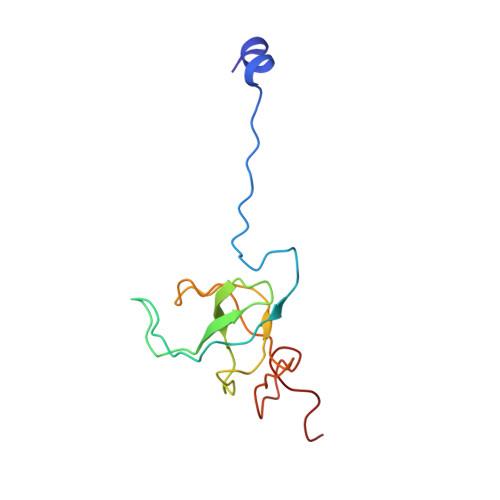
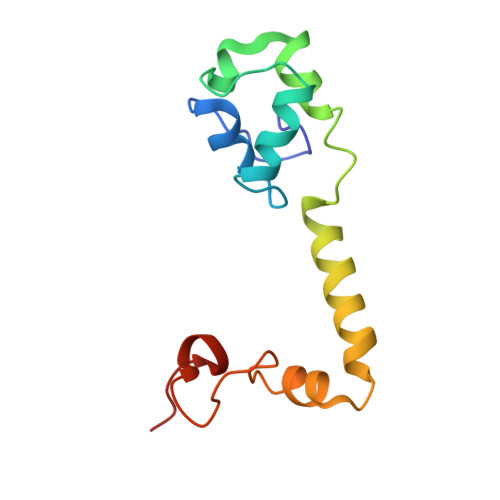
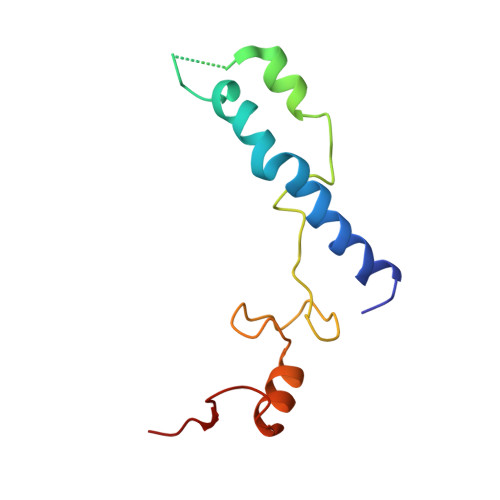
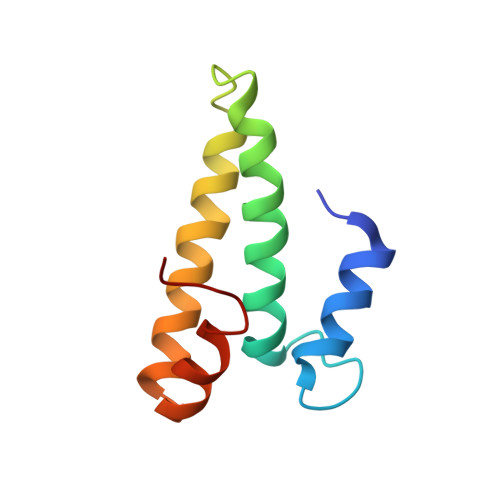
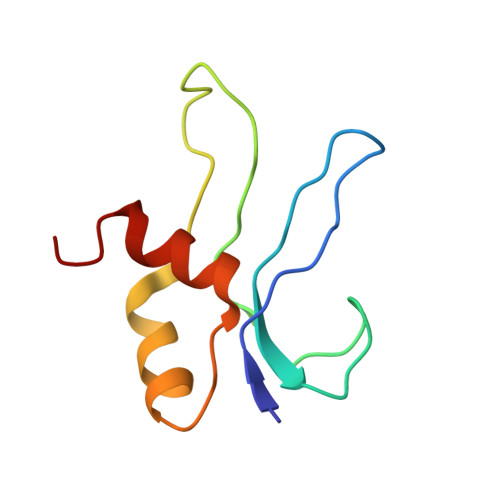
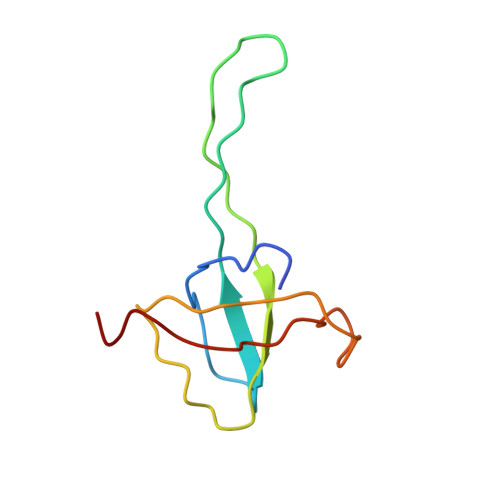
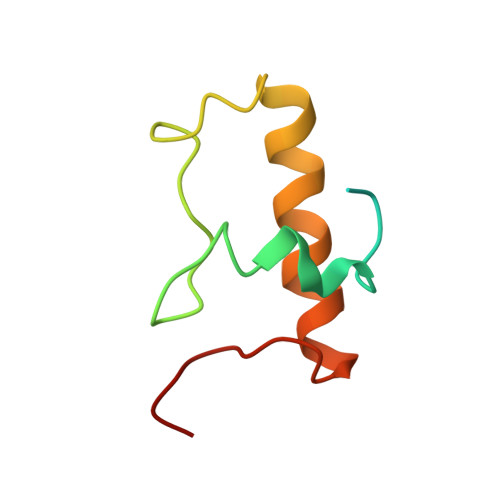
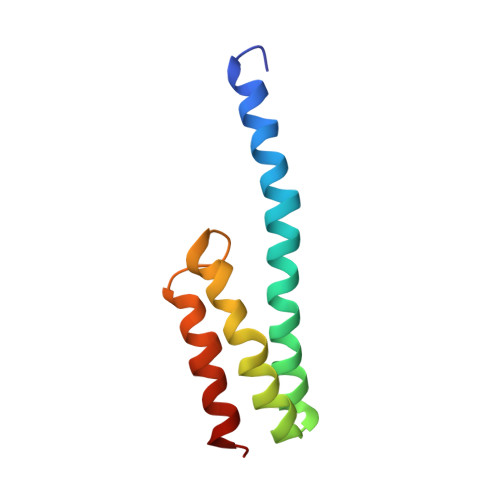
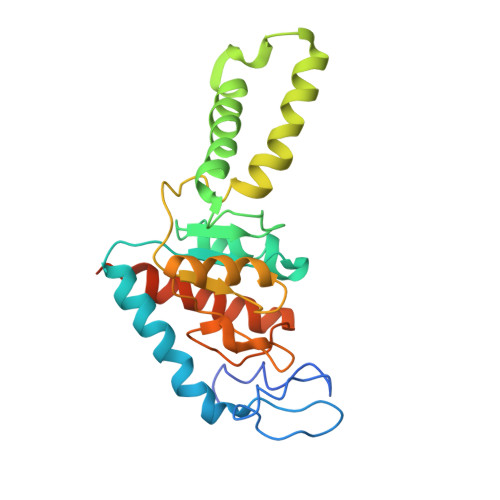
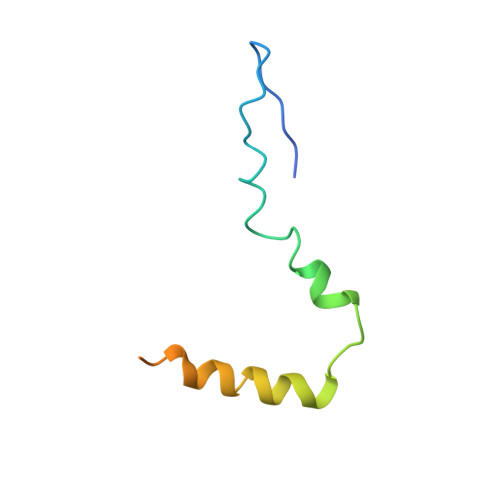
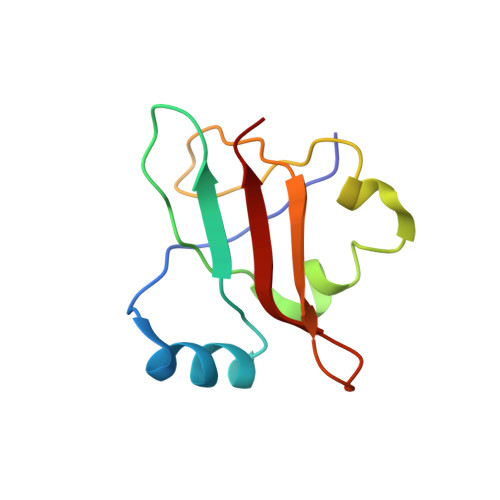
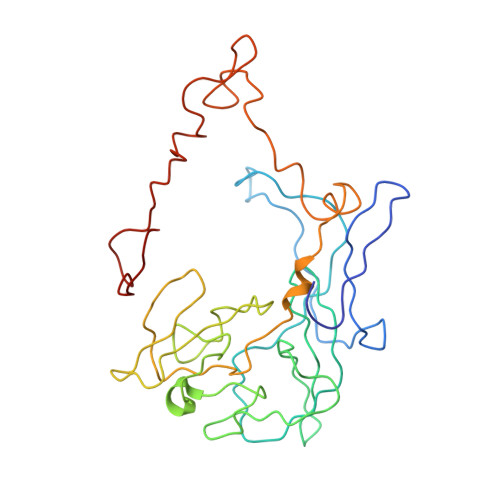
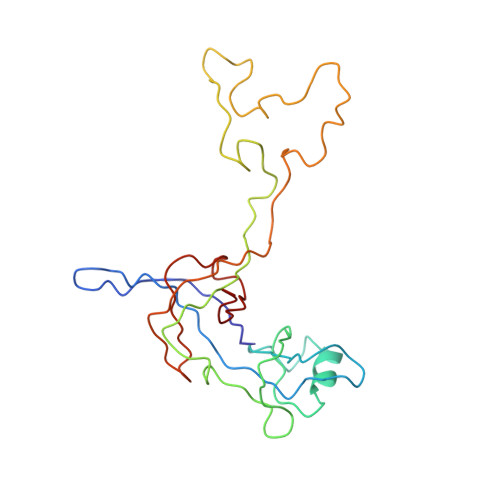
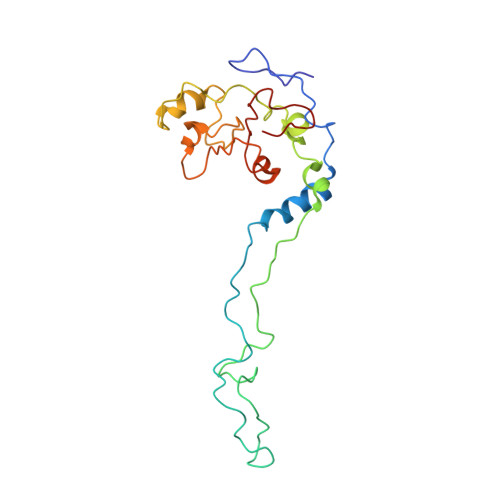
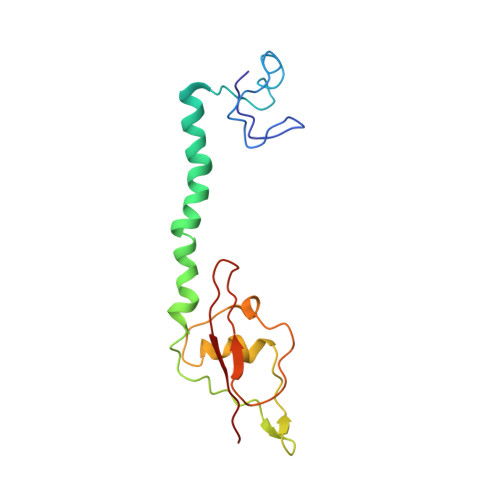
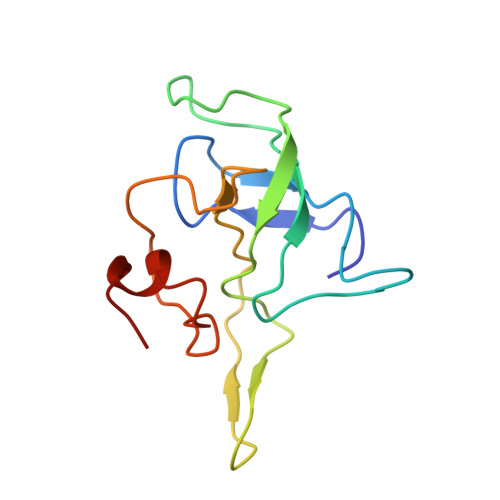
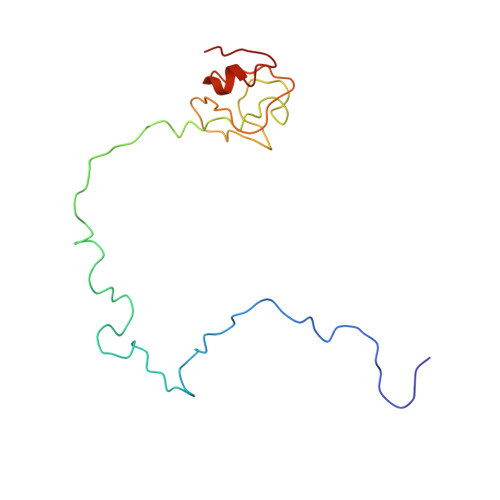
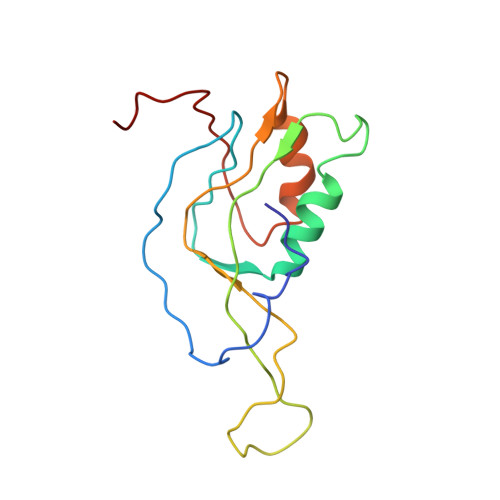
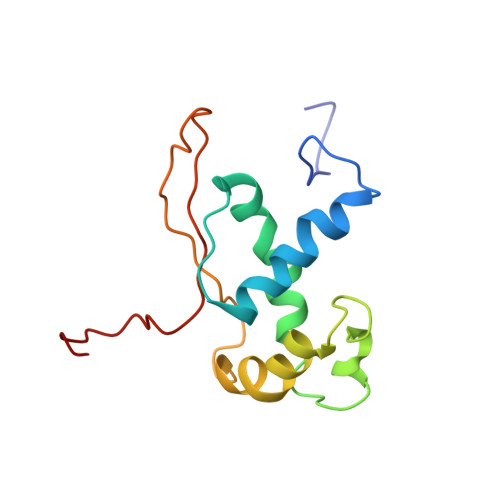
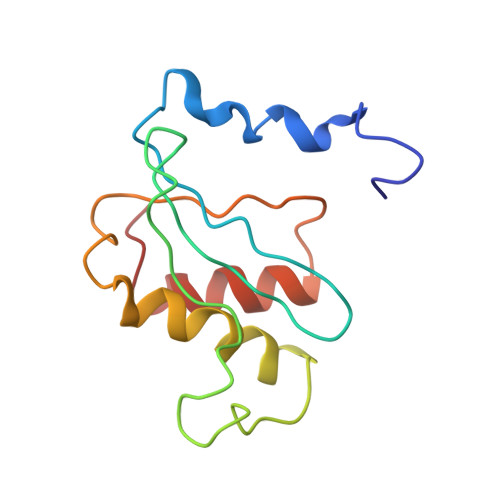
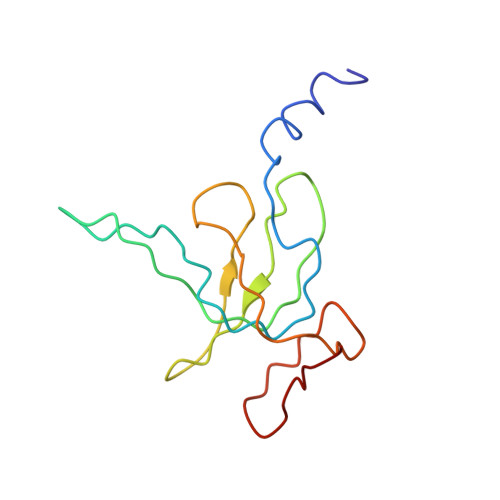
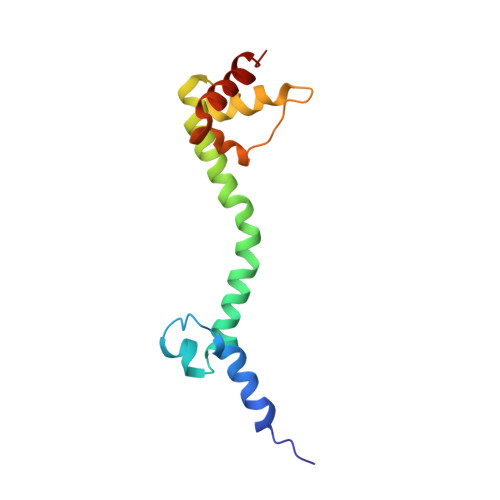
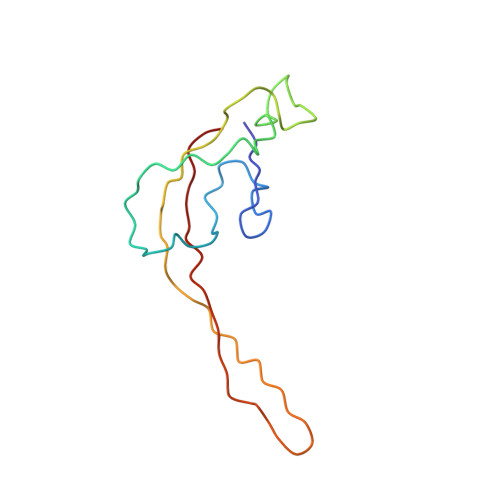
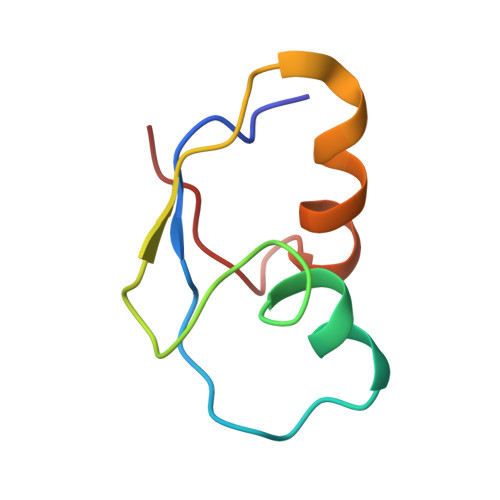
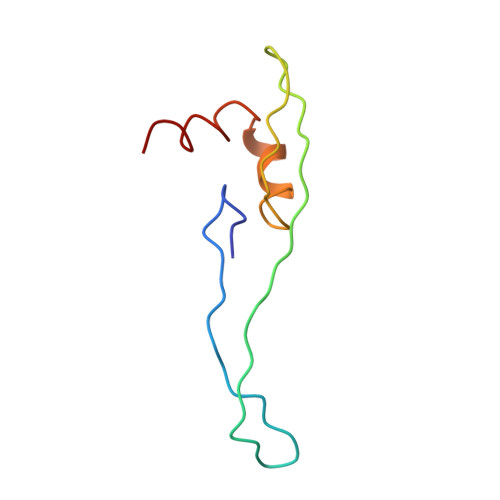
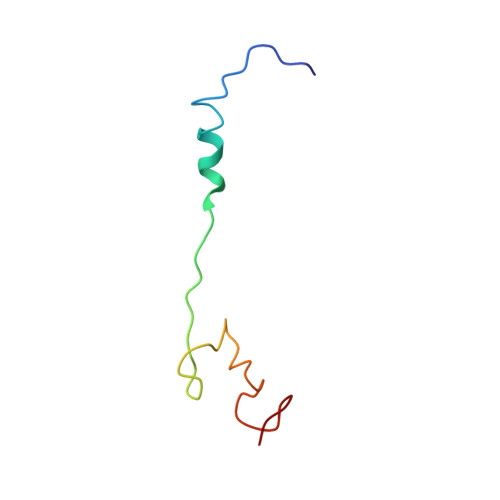
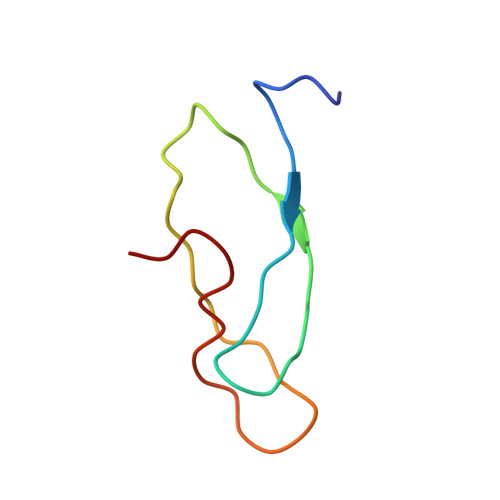
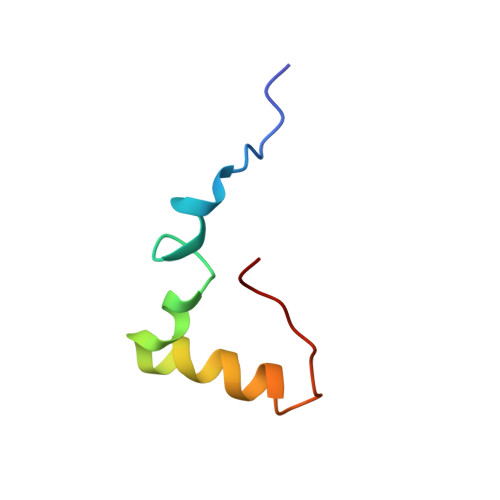
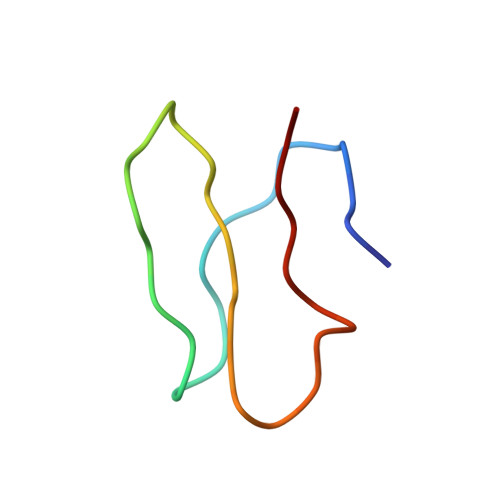
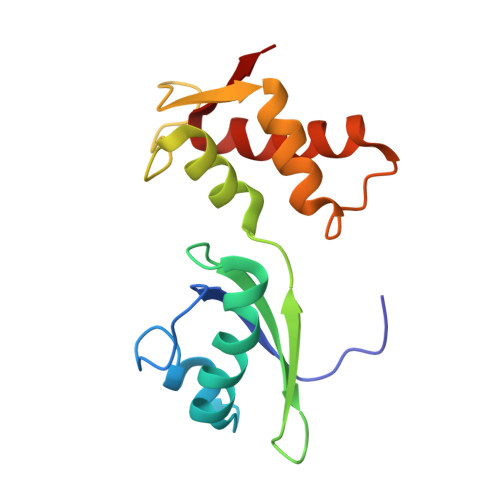
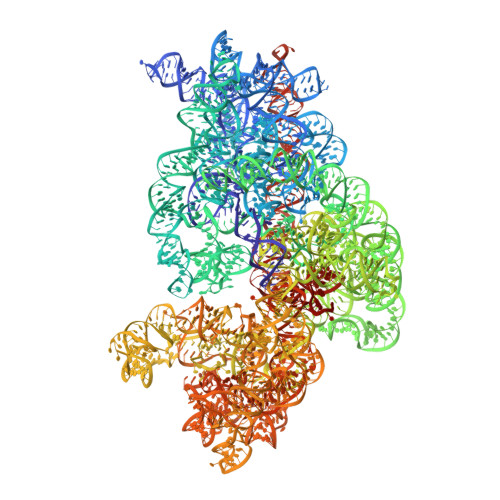
MG Query on MG Download Ideal Coordinates CCD File AD [auth AA]
AD [auth AA],
MAGNESIUM ION