The First Crystal Structure of the UP1 Domain of hnRNP A1 Bound to RNA Reveals a New Look for an Old RNA Binding Protein.
Morgan, C.E., Meagher, J.L., Levengood, J.D., Delproposto, J., Rollins, C., Stuckey, J.A., Tolbert, B.S.(2015) J Mol Biology 427: 3241-3257
- PubMed: 26003924
- DOI: https://doi.org/10.1016/j.jmb.2015.05.009
- Primary Citation of Related Structures:
4YOE - PubMed Abstract:
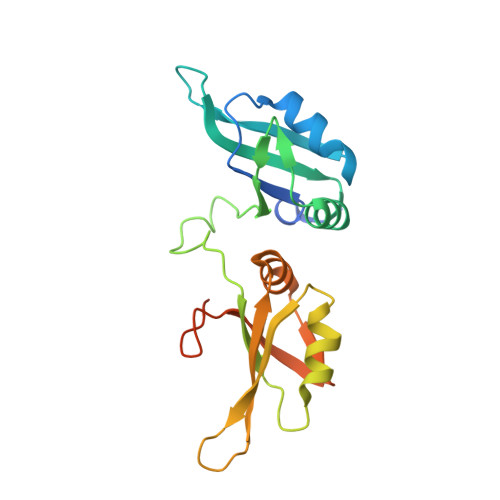
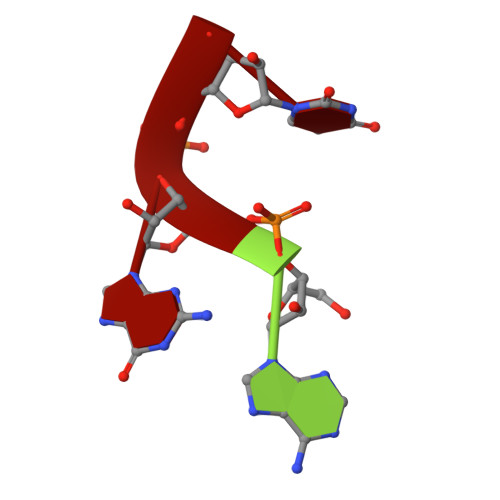
The heterogeneous nuclear ribonucleoprotein (hnRNP) A1 protein is a multifunctional RNA binding protein implicated in a wide range of biological functions. Mechanisms and putative hnRNP A1-RNA interactions have been inferred primarily from the crystal structure of its UP1 domain bound to ssDNA. RNA stem loops represent an important class of known hnRNP A1 targets, yet little is known about the structural basis of hnRNP A1-RNA recognition. Here, we report the first high-resolution structure (1.92Å) of UP1 bound to a 5'-AGU-3' trinucleotide that resembles sequence elements of several native hnRNP A1-RNA stem loop targets. UP1 interacts specifically with the AG dinucleotide sequence via a "nucleobase pocket" formed by the β-sheet surface of RRM1 and the inter-RRM linker; RRM2 does not contact the RNA. The inter-RRM linker forms the lid of the nucleobase pocket and we show using structure-guided mutagenesis that the conserved salt-bridge interactions (R75:D155 and R88:D157) on the α-helical side of the RNA binding surface stabilize the linker in a geometry poised to bind RNA. We further investigated the structural basis of UP1 binding HIViSL3(ESS3) by determining a structural model of the complex scored by small-angle X-ray scattering. UP1 docks on the apical loop of SL3(ESS3) using its RRM1 domain and inter-RRM linker only. The biophysical implications of the structural model were tested by measuring kinetic binding parameters, where mutations introduced within the apical loop reduce binding affinities by slowing down the rate of complex formation. Collectively, the data presented here provide the first insights into hnRNP A1-RNA interactions.
- Department of Chemistry, Case Western Reserve University, Cleveland, OH 44106, USA.
Organizational Affiliation: