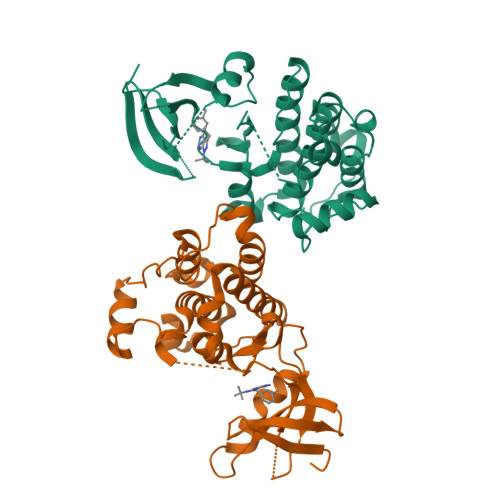
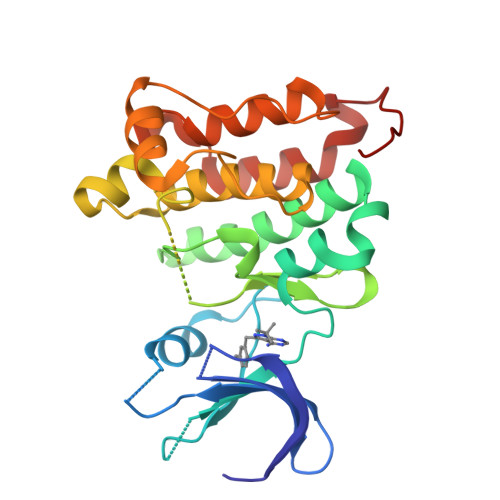
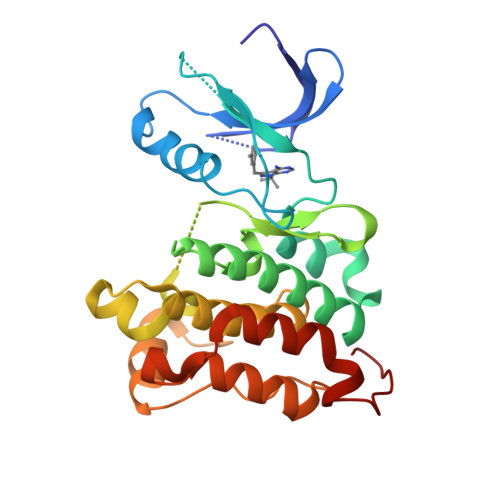
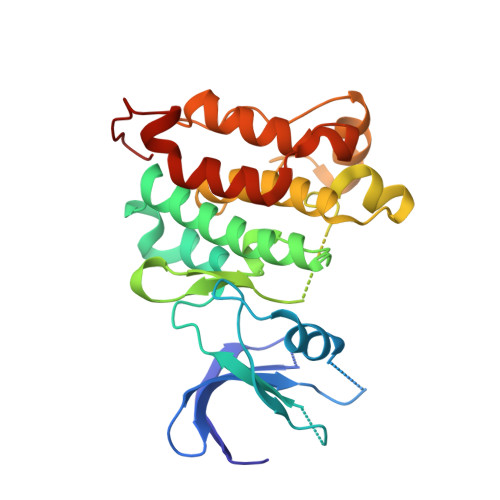
Structure-guided inhibitor design expands the scope of analog-sensitive kinase technology.
Zhang, C., Lopez, M.S., Dar, A.C., Ladow, E., Finkbeiner, S., Yun, C.H., Eck, M.J., Shokat, K.M.(2013) ACS Chem Biol 8: 1931-1938
- PubMed: 23841803
- DOI: https://doi.org/10.1021/cb400376p
- Primary Citation of Related Structures:
4LGG, 4LGH - PubMed Abstract:
Engineered analog-sensitive (AS) protein kinases have emerged as powerful tools for dissecting phospho-signaling pathways, for elucidating the cellular function of individual kinases, and for deciphering unanticipated effects of clinical therapeutics. A crucial and necessary feature of this technology is a bioorthogonal small molecule that is innocuous toward native cellular systems but potently inhibits the engineered kinase. In order to generalize this method, we sought a molecule capable of targeting divergent AS-kinases. Here we employ X-ray crystallography and medicinal chemistry to unravel the mechanism of current inhibitors and use these insights to design the most potent, selective, and general AS-kinase inhibitors reported to date. We use large-scale kinase inhibitor profiling to characterize the selectivity of these molecules as well as examine the consequences of potential off-target effects in chemical genetic experiments. The molecules reported here will serve as powerful tools in efforts to extend AS-kinase technology to the entire kinome and the principles discovered may help in the design of other engineered enzyme/ligand pairs.
Organizational Affiliation:
Howard Hughes Medical Institute and Department of Cellular & Molecular Pharmacology, University of California, San Francisco , San Francisco, California 94158, United States.