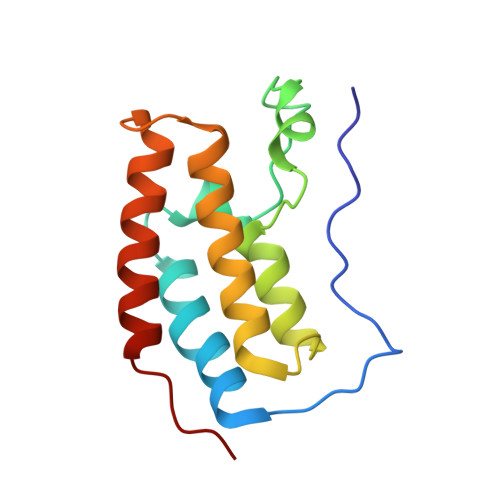
Crystal structure of BRD4 first bromodomain in complex with a 3,5-dimethylisoxazol ligand
Tallant, C., Hay, D., Krojer, T., Nunez-Alonso, G., Picaud, S., Newman, J.A., Fedorov, O., von Delft, F., Arrowsmith, C.H., Edwards, A.M., Bountra, C., Brennan, P.E., Knapp, S.To be published.